CRC Handbook of Chemistry and Physics, 91Th Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bromo Cresol Green Indicator I002
Bromo Cresol Green Indicator I002 Bromocresol green (BCG) indicator is used as a pH indicator in applications such as growth mediums for microorganisms and titrations. Composition** Ingredients Bromocresol green sodium salt 0.04 gm Disilled water 100.0ml **Formula adjusted, standardized to suit performance parameters Principle And Interpretation Bromocresol green (BCG) is a dye of the triphenylmethane family (triarylmethane dyes). The compound is synthesized by bromination of cresol purple (m-cresolsulfonphthalein). In aqueous solution, Bromocresol green will ionize to give the monoanionic form (yellow), that further deprotonates at higher pH to give the dianionic form (blue), which is stabilized by resonance. The Dissociation constant (pKa) of this reaction is 4.8. It becomes yellow at acidic pH level (pH 3.8) and Blue - green at from pH 5.4 . Quality Control Appearance Green coloured solution. Clarity Clear without any particles. Reaction At pH 3.8, the indicator turns yellow and at pH 5.4, the indicator is blue-green. Storage and Shelf Life Store between 10- 30°C. Use before expiry date on the label. Reference 1.Fred Senese. "Acid-Base Indicators". Frostburg State University Dept. of Chemistry. 2.Chemistry infolab reagents and resources ; The preparation of titration indicators; Dhanal De Lloyd,chem.Dept Revision : 1 / 2015 Disclaimer : User must ensure suitability of the product(s) in their application prior to use. Products conform solely to the information contained in this and other related HiMedia™ publications. The information contained in this publication is based on our research and development work and is to the best of our knowledge true and accurate. -

Bromocresol Green
Catalog Number: 101119, 152502, 152503, 195079, 806346, 806348 Bromocresol Green Structure: (sodium salt) Free Acid Sodium Salt Molecular Formula: C21H14Br4O5S C21H13Br4O5SNa Molecular Weight: 698.0 720 CAS # 76-60-8 62625-32-5 Synonyms: 3',3'',5',5''-Tetrabromo-m-cresolsulfonephthalein; Bromcresol Green Physical Description: Yellow to tan powder (free acid); Reddish-brown to Greenish-black crystalline powder (sodium salt) Solubility: Free Acid: Soluble in water (6 mg/ml), ethanol (40 mg/ml) or methyl cellosolve (200 mg/ml) Sodium Salt: Soluble in water (40 mg/ml) or ethanol (60 mg/ml). Description: Tracking dye for DNA agarose electrophoresis. lmax = 617 (400) nm in water. Also used as a pH indicator (3.8 to 5.4 : yellow to blue-green). Bromocresol green binds quantitatively with human albumin1 forming an intense blue-green complex with an absorbance maximum at 628 nm. The intensity of the color produced is directly proportional to the albumin concentration in the sample. Availability: Catalog Number Description Size 101119 Bromocresol Green 1 g 5 g 25 g 152502 Bromocresol Green, ACS Grade 1 g 5 g 25 g 152503 Bromocresol Green, sodium salt, ACS 1 gm Grade 5 gm 25 gm 195079 Bromocresol Green, sodium salt 1 gm 5 gm 10 gm 25 gm 806346 Bromocresol Green, sodium salt 5 gm 806348 10 gm References: 1. Kessler, M.A., et al., Anal. Biochem., v. 248, 180 (1997). 2. "Interaction of bromocresol green with different serum albumins studied by fluorescence quenching." Biochem. Mol. Biol. Int., v. 43:1, 1-8 (1997). 3. "Albumin standards and the measurement of serum albumin with bromocresol green. -

Application of Bromocresol Green and Bromothymol Blue for the Extractive Spectrophotometric Determination of Anti-Hypertensive Drugs
Journal of Applied Pharmaceutical Science Vol. 5 (07), pp. 122-129, July, 2015 Available online at http://www.japsonline.com DOI: 10.7324/JAPS.2015.50719 ISSN 2231-3354 Application of Bromocresol Green and Bromothymol Blue for the Extractive Spectrophotometric Determination of Anti-hypertensive Drugs Akram M. El-Didamony1, Sameh M. Hafeez2, Ahmed A. Saad1 1 Chemistry Department, Faculty of Science, Zagazig University, Zagazig 44519, Egypt. 2 Ismailia Chemical Laboratory, Forensic Medicine Authority, Justice Ministry, Egypt. ABSTRACT ARTICLE INFO Article history: Simple, selective and highly sensitive spectrophotometric methods are proposed for the rapid and accurate Received on: 10/04/2015 determination of anti-hypertensive drugs namely telmisartan (TEL), propranolol (PRO), bisoprolol (BIS) and Revised on: 02/05/2015 carvedilol (CRV) in tablets and biological fluids using bromocressol green (BCG) and bromothymol blue (BTB). Accepted on: 16/06/2015 The developed methods involve formation of stable yellow colored dichloromethane extractable ion-pair Available online: 27/07/2015 complexes of the amino derivative of four antihypertensive drugs such as TEL, PRO, BIS and CRV with two sulphonphthalein acid dyes, namely; BCG and BTB in acidic buffer. The effect of optimum conditions via pH on Key words: the ion-pair formation, reagent concentration, time and temperature and solvent was studied. The composition of Antihypertensive drugs, the ion-pairs was found 1: 1 by Job’s method. The established methods having high sensitivity and good Extraction spectrophotometry, selectivity could be applied to the determination of the studied drugs in pharmaceutical, urine and blood serum BCG and BTB dyes; Ion-pair samples with satisfactory results. -

Bromocresol Purple
Bromocresol Purple Catalog Number: 150518, 195080 Bromocresol Purple Structure :(free acid) Free Acid Sodium Salt Molecular Formula: C21H16Br2O5S C21H15Br2O5SNa Molecular Weight: 540.2 562.2 CAS # 115-40-2 62625-30-3 Synonym: 5',5''-Dibromo-o-cresolsulfonephthalein Physical Description: Reddish-brown crystalline powder (sodium salt); Orange to purplish-brown crystalline powder (free acid) pK: 6.3 Solubility: Free Acid: Soluble in water (20 mg/ml), ethanol (80 mg/ml) or methyl cellosolve (300 mg/ml). Sodium Salt: Soluble in water (80 mg/ml), ethanol (20 mg/ml) or methyl cellosolve (90 mg/ml). Description: Typically used as a pH indicator (5.2-6.8 : yellow to blue-purple). Bromocresol purple binds quantitatively with human serum1 albumin forming a stable complex with absorbance maximum at 600 nm. The intensity of the color produced is directly proportional to the albumin concentration in the sample. Availability: Catalog Number Description Size 195080 Bromocresol Purple 5 g 25 g 100 g 150518 Bromocresol Purple, Sodium Salt 5 g 25 g References: 1. Hill, P.G. and Wells, T.N.C., Ann. Clin. Biochem., v. 20, 264 (1983). 2. Orndorff, W.R. and Purdy, A.C., J. Am. Chem. Soc., v. 48, 2216 (1926). file:///C|/Users/Administrator/Desktop/新建文件夹/150518.htm[2013/3/4 13:44:01] Bromocresol Purple 3. "Reduction of reaction differences between human mercaptalbumin and human nonmercaptalbumin measured by the bromocresol purple method." Clin. Chim. Acta, v. 289:1-2, 69-78 (1999). 4. "A new photometric assay with bromocresol purple for testing in vitro antitrichomonal activity in aerobic environment." Arzneimittelforschung, v. -

Datasheet for Tridye™ 100 Bp DNA Ladder (N3271; Lot 0131204)
restriction enzymes to yield 12 bands suitable for Base Pairs Mass (ng) ™ TriDye Relative Migration Rates (approximate) TriDye 100 bp use as molecular weight standards for agarose 1,517 45 gel electrophoresis. The digested DNA includes % agarose xylene cyanol FF bromophenol blue orange G DNA Ladder fragments ranging from 100–1,517 base pairs. 0.5 20–40 kb 4,000 bp 150 bp 1,200 35 The 500 and 1,000 base pair bands have increased intensity to serve as reference points. 0.8 8,000 bp 400 bp 75 bp 1,000 95 1-800-632-7799 900 27 [email protected] www.neb.com Supplied in: 0.006% xylene cyanol FF, 0.006% 1.0 4,000 bp 300 bp 50 bp 800 24 N3271S 013120414041 700 21 bromophenol blue, 0.06% orange G, 10% glycerol, 1.3 1,800 bp 150 bp 15 bp 10 mM Tris-HCl (pH 7.9) and10 mM EDTA. 600 18 1.5 1,200 bp 100 bp 10 bp N3271S TriDye During Electrophoresis 500/517 97 2.0 700 bp 65 bp < 10 bp 125 gel lanes (1.25 ml) Lot: 0131204 50 µg/ml 400 38 Store at 4°C Exp: 4/14 xylene cyanol FF 100 bp DNA Ladder 300 29 Usage Recommendation: We recommend loading ™ visualized by Description: TriDye 100 bp DNA Ladder is a 10 µl (0.5 µg) of TriDye 100 bp DNA Ladder per ethidium bromide 200 25 pre-mixed, ready-to-load molecular weight bromophenol blue gel lane. The TriDye 100 bp DNA Ladder was not staining on a 1.3% marker containing 3 dyes which serve as visual TAE agarose gel. -

Kinetic and Thermodynamic Study of the Reaction Between Bromocresol Green with Sodium Hydroxide in an Aqueous Solution
JournalJournal of Chemical of Chemical Technology Technology and and Metallurgy, Metallurgy, 54, 54,1, 2019, 1, 2019 90-94 KINETIC AND THERMODYNAMIC STUDY OF THE REACTION BETWEEN BROMOCRESOL GREEN WITH SODIUM HYDROXIDE IN AN AQUEOUS SOLUTION Latona Dayo Felix1, Adejoro Ajibade2 1 Department of Chemical Sciences, Osun State University Received 15 November 2017 PMB 4494 Osogbo, Nigeria Accepted 06 March 2018 2 Department of Chemistry, University of Ibadan, Nigeria E-mail: [email protected] ABSTRACT The kinetics and the mechanism of the reaction of Bromocresol green and a hydroxyl ion in an aqueous solution is studied spectrophotometrically. The reaction is found of a first order in respect to both reactants. The Michaelis-Menten plot shows intermediate complex presence and reaction dependence on the solution ionic strength. The activation param- eters are evaluated. The negative ΔS‡ value obtained indicates an associative mechanism. Keywords: kinetics, thermodynamics, mechanism, Bromocresol green (BCG), sodium hydroxide and potassium nitrate. INTRODUCTION to emptying them into the water bodies as industrial ef- fluents is imminent in order to protect the aquatic organ- Bromocresol green belongs to the triphenylmethane isms [3]. Several methods of achieving this noble objec- dye family. It is an important industrial raw material tive are reported [4-7]. However, this research tends to in textile, leather, paper, printing, plastic and ceramic investigate the option of hydrolyzing Bromocresol green industries [1]. Bromocresol green is employed in col- in an alkaline medium with the view to ascertaining the orimetric detection technologies and for visualization of kinetics and the mechanism of the reaction, which has compounds of a functional group whose pKa is below hitherto not been reported in the literature. -

ORANGE G SODIUM SALT Molecular Biology Reagent Product No
ORANGE G SODIUM SALT Molecular Biology Reagent Product No. O3756 Store at room temperature Product Summary Endonuclease-Exonuclease Suitable for use as a tracking dye for nucleic acid gel One mg of l Hind III fragments was incubated for 16 hours electrophoresis at 37 °C with orange G at a final concentration of 0.18% DNase, RNase: None detected (w/v) in a 50 ml reaction mixture containing 30 mM Ò Mol. Wt. 452.4 Trizma -HCl, pH 7.8, 50 mM NaCl and 10 mM MgCl2. No degradation of the DNA fragments was detected by Suitability agarose gel electrophoresis. Detection limit: One volume dye solution (30% (w/v) sucrose and Degradation of 10% of the DNA substrate is detectable. 0.35% (w/v) orange G) was added to five volumes sample containing pBR322/Hae III DNA. Upon electrophoresis RNase (4% wide-range agarose gel) the orange G was found to Two mg of transfer RNA were incubated with orange G at run faster than bromophenol blue and the smallest a final concentration of 0.18% (w/v) in a 50 ml reaction detectable DNA fragment (approx. 51 bp). The dye front mixture containing 30 mM TrizmaÒ-HCl, pH 7.8, 50 mM was plainly visible during the course of the NaCl and 10 mM MgCl2 for 16 hours at 37 °C. No electrophoresis. degradation of the tRNA was detected by polyacrylamide gel electrophoresis. Detection limit: Degradation of 10% Endonuclease (Nickase) of the tRNA substrate is detectable. One mg of pBR322 DNA was incubated with orange G at a final concentration of 0.18% (w/v) in a 50 ml reaction 12/97 mixture containing 30 mM TrizmaÒ-HCl, pH 7.8, 50 mM NaCl and 10 mM MgCl2 for 16 hours at 37 °C. -

A Simplified Method for Finding the Pka of an Acid–Base Indicator by Spectrophotometry
In the Laboratory A Simplified Method for Finding the pKa of an Acid–Base Indicator by Spectrophotometry George S. Patterson* Suffolk University, 41 Temple Street, Boston, MA 02114 λ General chemistry textbooks devote much space to the max at a different wavelength. If the cell path length is kept important concept of equilibrium. To illustrate one aspect constant and all solutions contain the same total molarity of λ of equilibrium, a new laboratory experiment on the measure- indicator, the acid–base ratio at the max of either the acid or ment of an equilibrium constant was desired. Ideally, the ex- base form is given by (3, 10, 11) periment would { 1. result in a reasonably accurate value of the equilibrium HIn A ± AIn constant, { = (2) [In ] AHIn ± A 2. use a small number of solutions that are safe to handle—or at least be familiar to students—and simple to dispose, where A is the absorbance of the solution containing a certain total concentration of the acid–base mixture, A { is the 3. use equipment commonly found in a general chemistry In laboratory, and absorbance of the base form at the same concentration, and AHIn is the absorbance of the acid form at the same concentration. 4. not exceed the skills of a typical general chemistry Substituting the expression for the HIn–In{ ratio from student. eq 2 into eq 1, A well-known experiment in analytical and physical { chemistry laboratory courses is the spectrophotometric A ± AIn pK = pH + log (3) determination of the pK of an acid–base indicator (1–9). -

5X Loading Buffer Blue Quality Control Each Lot of Loading Buffer Is Functionally Tested on an Agarose Gel
5x Loading Buffer blue Quality control Each lot of Loading Buffer is functionally tested on an agarose gel. Cat. No.: 733-2575 How to choose the right loading buffer Cap Cat. No. Product ID No. Volume colour Tracking dyes The choice of the tracking dye is dependent on the size of 5x Loading Buffer 733-2575 5400200 Blue 5 x 1 ml the DNA fragments one wants to run. In general, the front blue of the tracking dye should not run at the size of the DNA fragments because especially dark dyes obscure the DNA Store at –20 °C Reagent for in vitro laboratory use only bands. It is best to choose a tracking dye that runs in front of the DNA fragments to analyze. Features Suitable for agarose and SDS DNA gels Suitable for TAE, TBE, SB and LB electrophoresis White UV buffers 5x ready-to-use formulation Gel slots Ficoll-based for convenient storage on RT 1000 General Description 500 Cresol Red 300 DNA loading buffers are used to load DNA samples to Bromophenol Blue agarose or SDS DNA gels for gel electrophoresis. 100 Orange G DNA loading buffers serve three main purposes: Firstly, loading buffers add density to the DNA samples, so the DNA sinks down into the well instead of floating up and mixing with the running buffer. To achieve this, high density reagents like glycerol, sucrose or Ficoll are added. Figure 1: The three VWR Loading Buffers were run on a Secondly, loading buffers add visibility to the DNA sample. Loading buffers contain a coloured tracking dye to allow 1 % agarose gel along with a DNA marker. -

Basic Biotechnology Kit GEL ELECTROPHORESIS of DYES
Basic Biotechnology Kit GEL ELECTROPHORESIS OF DYES Partnership for Biotechnology and Genomics Education Barbara Soots Linda Curro Education Coordinator Assistant Education Coordinator University of California Davis University of California Davis 530 752‐6552 530 752‐6613 530 754‐4410 (fax) 530 754‐4410 (fax) [email protected] [email protected] This program is made possible through the generous support of: Teacher GEL ELECTROPHORESIS Information Dye Samples Introduction ¾ electrophoresis gel box In this experiment, students will be using ¾ 1 gel tray with 6 tooth comb agarose gel electrophoresis to separate several ¾ 50 ml beaker or graduated cylinder * dyes⎯ a precursor to the DNA separations we ¾ disposable plastic pipette will perform in later labs. ¾ 250 ml beaker * ¾ 20 µl micropipet with tips This lab will help students gain familiarity with ¾ gloves equipment and techniques essential to ¾ safety glasses * biotechnology. They will learn to cast and load ¾ paper to cover lab bench * an agarose gel as well as correctly utilize gel electrophoresis equipment. Attached readings and activities will better illustrate this important Common Materials technique. ¾ power supplies ¾ 60°C water bath Note: Your students should have completed the Micropipet Technique: Dye Samples lab prior ¾ 0.7% agarose solution (enough for 25 to running this experiment. ml per team) ¾ plastic carboy with TBE buffer (1X) ¾ gel disposal bags Objectives ¾ distilled water * 1. Understand the principles behind gel * Materials provided by instructor. electrophoresis. 2. Become familiar with preparing, loading and running a gel. Advance Preparation 3. Determine the effect of a molecule's electric charge and size on its movement 1. Dilute the concentrated TBE (10X) solution through an agarose gel. -
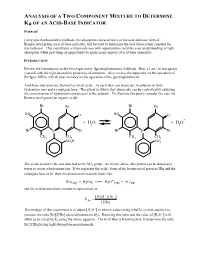
DETERMINATION of Ka of an ACID-BASE INDICATOR
ANALYSIS OF A TWO COMPONENT MIXTURE TO DETERMINE Ka OF AN ACID-BASE INDICATOR PURPOSE Using spectrophotometric methods, the absorption characteristics of the acid and base form of Bromocresol green, an acid-base indicator, will be used to determine the acid dissociation constant for this indicator. This experiment will provide you with opportunities to refine your understanding of light absorption while providing an opportunity to apply many aspects of acid-base chemistry. INTRODUCTION Review the Introduction to the first experiment “Spectrophotometric Methods: Beer’s Law” to reacquaint yourself with the light absorption properties of solutions. Also, review the Appendix on the operation of the Spec 20D to refresh your memory on the operation of the spectrophotometer. Acid-base indicators are themselves weak acids. As such they can dissociate in solution to form hydronium ions and a conjugate base. The extent to which they dissociate can be controlled by adjusting the concentration of hydronium ions present in the solution. To illustrate this point, consider the case for Bromocresol green (an organic acid): Br Br Br Br HO O HO O + + H2 O + H3 O Br C Br Br C Br CH3 CH3 CH3 CH3 - SO3 H SO3 The acidic proton is the one attached to the SO3 group. As shown above, this proton can be donated to water to create a hydronium ion. If we represent the acidic form of the bromocresol green as HIn and the conjugate base as In- then the dissociation reaction looks like: - HIn(aq) + H2O(l) <==> H3O+(aq) + In (aq) and the acid dissociation constant is represented as: [H O+ ][In− ] K = 3 In [HIn] + The strategy of this experiment is to adjust [H3O ] to known values using a buffer system and then to - + measure the ratio [In ]/[HIn] spectrophotometrically. -

DNA Loading Buffer the DNA Loading Buffer Is Shipped at Ambient Temperature and Stored at –20Oc
Storage and Stability: DNA Loading Buffer The DNA Loading Buffer is shipped at ambient temperature and stored at –20oC. Expiry Batch No.: See vial Product Catalog number When stored under the recommended conditions and handled correctly, full activity of the kit is retained until the expiry date on the outer box label. 5x Loading Buffer Blue BIO-37045: 2 x 1ml Safety precautions: This product is for R&D use only, not for human use, or any other use. Please refer to the 5x Loading Buffer Red BIO-37068: 2 x 1ml material safety data sheet for information regarding hazards and safe handling practice. 5x Loading Buffer Tri-Color BIO-37070: 2 x 1ml Quality control specifications: DNA Loading Buffer is extensively tested for batch-to-batch reproducibility prior to release. Trademarks MyTaq and HyperLadder are trademarks of Bioline Reagents Limited. Notes Research Use Only. Features: Applications: Colored loading for easy recognition To monitor migration rate during agarose electrophoresis No need to add dye Loading of samples onto DNA agarose gels Guarantee reproducible results Description The Colored DNA Loading Buffers are ready-to-use solutions premixed with bromophenol blue (Blue), cresol red (Red), orange G and xylene cyanol FF (TriColor). The dyes in the Colored DNA Loading Buffers migrate at different rates depending on the dye and the concentration of the agarose gel (see Dye Migration Table below). This allow users to monitor DNA migration, and therefore increase the versatility of their DNA analysis, by choosing the buffer most suited to their application. Recommended protocol 1. Add 1 volume of loading buffer to 4 volumes of sample 2.