Competitive Mating, Mate Choice and Mating Associations of Brachyuran Crabs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sydney Harbour: What We Do and Do Not Know About a Highly Diverse Estuary
Marine and Freshwater Research 2015, 66, 1073-1087 © CSIRO 2015 http://dx.doi.org/10.1071/MF15159_AC Supplementary material Sydney Harbour: what we do and do not know about a highly diverse estuary E. L. JohnstonA,B, M. Mayer-PintoA,B, P. A. HutchingsC, E. M. MarzinelliA,B,D, S. T. AhyongC, G. BirchE, D. J. BoothF, R. G. CreeseG, M. A. DoblinH, W. FigueiraI, P. E. GribbenB,D, T. PritchardJ, M. RoughanK, P. D. SteinbergB,D and L. H. HedgeA,B AEvolution and Ecology Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia. BSydney Institute of Marine Science, 19 Chowder Bay Road, Mosman, NSW 2088, Australia. CAustralian Museum Research Institute, Australian Museum, 6 College Street, Sydney, NSW 2010, Australia. DCentre for Marine Bio-Innovation, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia. ESchool of GeoSciences, The University of Sydney, Sydney, NSW 2006, Australia. FCentre for Environmental Sustainability, School of the Environment, University of Technology, Sydney, NSW 2007, Australia. GNew South Wales Department of Primary Industries, Port Stephens Fisheries Institute, Nelson Bay, NSW 2315, Australia. HPlant Functional Biology and Climate Change Cluster, University of Technology, Sydney, NSW 2007, Australia. ICentre for Research on Ecological Impacts of Coastal Cities, School of Biological Sciences, University of Sydney, NSW 2006, Australia. JWater and Coastal Science Section, New South Wales Office of Environment and Heritage, PO Box A290, Sydney, NSW 1232, Australia. KCoastal and Regional Oceanography Lab, School of Mathematics and Statistics, University of New South Wales, NSW 2052, Australia. -

Tf^A^(J€, W^ COLLINS-EVOLUTION of ANOMALOCARIS
f ^v^ Tf^A^(j€, W^ COLLINS-EVOLUTION OF ANOMALOCARIS . 1911a. Middle Cambrian Merostomata. Cambrian geology and Middle Cambrian, Bxu^ess Shale, British Columbia. Philosophical paleontology II. Smithsonian Miscellaneous Collections, 57:17-40. Transactions of the Royal Society of London, B271:1-43. 1911b. Middle Cambrian Holothurians and Medusae. Cambrian , AND D. E. G. BRIGGS. 1982. A new conundrum from the Middle geology and paleontology II. Smithsonian Miscellaneous Collections, Cambrian Burgess Shale. Proceedings of the Third North American 57:41-68. Paleontological Convention, Montreal, 2:573-575. 1911c. Middle Cambrian Annelids. Cambrian geology and pa , AND . 1985. The largest Cambrian animal, Anomalocaris, leontology II. Smithsonian Miscellaneous Collections, 57:109-144. Burgess Shale, British Coliunbia. Philosophical Transactions of the . 1912. Middle Cambrian Branchiopoda, Malacostraca, Trilobita Royal Society of London, B309:569-609. and Merostomata. Cambrian geology and paleontology II. Smithson WOODWARD, H. 1902. The Canadian Rockies. Part I: On a collection ian Miscellaneous Collections, 57:145-228. of Middle Cambrian fossils obtained by Edward Whymper, Esq., WmxEAVES, J. F. 1892. Description of a new genus and species of F.R.G.S., from Mount Stephen, British Columbia. Geological Mag Phyllocarid Crustacea from the Middle Cambrian of Moimt Stephen, azine, 4(9):502-505, 529-544. B.C. Canadian Record of Science, 5:205-208. WHrmNGTON, H. B. 1975. The enigmatic animal Opabinia regalis, ACX:EPTED 8 AUGUST 1995 /. Paleont., 70(2), 1996, pp. 293-296 Copyright © 1996, The Paleontological Society O022-3360/96/OO70-0293$03.0O A NEW TETHYAN MIGRANT: CRETACHLORODIUS ENCIENSISN, GEN., N. SP. (CRUSTACEA, DECAPODA), FROM THE MAASTRICHTIAKT^YPE AREA RENEH. -

Archiv Für Naturgeschichte
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Bericht über die Leistuugen in der Carcinologie wälirend des Jahres 1894. Von Dr. F. Hilgendorf und Dr. J. Vosseier*). Verzeichniss der Publicationen. Albert I,, Prince de Monaco: Sur les premieres campagnes de la princesse Alice. Compt. rend. Ac. Sc. Paris T. CXX. — Eine 2 m tief ins Meer eingesenkte Lampe lockt in kurzer Zeit ganze Wolken kleiner Kruster herbei. F. Albrecht, L. K., Ziornow u. a. Primitiae faunae Mosquensis. Congres intern, d'anthrop., arch. et zool. 1892 (Moscou), Materiaux i-eunis etc. 1. partie, Suppl. Nr. 16, 137 S.; Crust. p. 121 — 5. Mos- cou 1893. — 122 Entom. u. 14 Malacostraca, Alcock, A. Natural bist, notes from „Investigator" (Ser. 2) Nr. 1. (continued). (Vergl. Ber. 91, 92, 93 unter Wood-Mason, W.- M. u. Alcock, Alcock). Ann. Mag. (6) XIII p. 225-45, 321—34, 400—411. — Behandelt Deep-sea dredging 1890/91. Spec. Nr. 58 bis 99. Farn. Nematocarcinidae, Honiar., Eryont. (IXyl.), Parapagur., Galath., Inachidae, Cancridae (Platypilumyins)^ Ocypod. (Psopheticus), Leucos. {Ci/monomops), Homolidae. Stomatopoda (2 Sp.), Amphi- poda (l Sp., Farn. Stegoceph., Xyl). 28 neue Sp. od. Variet. Sperma- tozoen V. Munida besclir. p. 324. Stridulationsapp. bei Psophet. Rudim. Augen bei Cymon. u. Andania. Alcock, A. and A. R. Audeison (1). Nat. bist, notes from „Investigator" (2. Ser.) No. 14: An account of a recent coli, of deep sea Crustacea from the Bay of Bengal and Laccadive Sea. Journ. Asiat, soc. of Bengal, Vol. 63 part. IL No. 3. p. 141—185. Tfl. IX. *) Im Allgemeinen sind die Arbeiten über höhere Krebse von Hilgendorf, die über niedere von Vosseier besprochen worden, lieber etwaige Ausnahmen giebt die Unterzeichnung der betreif. -

Download Full Article 1.3MB .Pdf File
Memoirs of the National Museum of Victoria 12 April 1971 Port Phillip Bay Survey 2 https://doi.org/10.24199/j.mmv.1971.32.05 BRACHYURA (CRUSTACEA, DECAPODA) By D. J. G. Griffin and J. C. Yaldwyn* Australian Museum, Sydney Abstract The SurVey C0 Iected 102 specimens of Brachyura *a -| c ! ? belonging to 29 Species and 10 families.m Seven species were taken by the Portland Pier Survey in 1963 five of which are also represented in the Port Phillip Survey collection. Only four of the 38 species known m 3re re resent d the collection. P ? '? The majid Paratymolus talipes and the xanthidTamh-YPilumnuspf acer are recorded from Victoria for the first time; previous records of the graspid\Cyclograpsus audouinii from Victoria are doubtful. Seventeen species known from Port Phillip are not represented in the collection. All are typically cool temperate species well known from SE. Australia. Four species of Pilumnus were represented in the collections and these are compared in detail with other SE. Australian Pilumnus species. Most abundant in Port Phillip are Hahcaranus ovatus and H. rostratus (Hymenosomatidae) Notomithrax minor (Majidae), Ebalia (Phylyxia) intermedia (Leucosiidae), Lilocheira bispinosa (Gone- placidae), Pilumnus tomentosus and P. monilifer (Xanthidae), Nectocardnus integrifrons and Carcinus maenas (Portunidae) and Pinnotheres pisum (Pinnotheridae). The majority of the species are found on the sandy areas around the edge of the Bay, particularly in the W areas; no species was taken in the central deeper parts of the Bay. Ovigerous females of most species were collected in late summer. Parasitism by sacculinas was small and confined to two species of Pilumnus. -

Adaptations by the Locomotor Systems of Terrestrial and Amphibious Crabs Walking Freely on Land and Underwater
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2004 Adaptations by the locomotor systems of terrestrial and amphibious crabs walking freely on land and underwater Jennifer Nuss Schreiner Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Recommended Citation Schreiner, Jennifer Nuss, "Adaptations by the locomotor systems of terrestrial and amphibious crabs walking freely on land and underwater" (2004). LSU Master's Theses. 1349. https://digitalcommons.lsu.edu/gradschool_theses/1349 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. ADAPTATIONS BY THE LOCOMOTOR SYSTEMS OF TERRESTRIAL AND AMPHIBIOUS CRABS WALKING FREELY ON LAND AND UNDERWATER A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Science in The Department of Biological Sciences by Jennifer Nuss Schreiner B.S., Louisiana State University, 2001 August 2004 ACKNOWLEDGEMENTS I would like to begin by expressing my most heartfelt appreciation to Dr. Jim Belanger. Thank you for giving me the opportunity to be part of your laboratory family, for your everlasting faith in me, and for the endless hours of reassurance when it seemed nothing would ever go as planned. Your patience and generosity mean a great deal to me. -
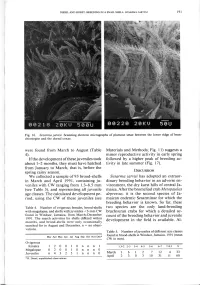
(Table 4). If the Development of These Juveniles Took About 1-2 Months
DIESEL AND HORST: BREEDING IN A SNAIL SHELL: SESARMA JARVISI 191 Fig. 16. Sesarma jarvisi. Scanning electron micrographs of plumose setae between the lower ridge of bran- chiostegite and the dorsal coxae. were found from March to August (Table Materials and Methods; Fig. 11) suggests a 4). minor reproductive activity in early spring If the development of these juveniles took followed by a higher peak of breeding ac- about 1-2 months, they must have hatched tivity in late summer (Fig. 17). from January to March, that is, before the spring rainy season. DISCUSSION We collected a sample of 93 brood-shells Sesarma jarvisi has adopted an extraor- in March and April 1991, containing ju- dinary breeding behavior in an adverse en- veniles with CW ranging from 1.3-8.5 mm vironment, the dry karst hills of central Ja- (see Table 5), and representing all juvenile maica. After the bromeliad crab Metopaulias age classes. The calculated development pe- depressus, it is the second species of Ja- riod, using the CW of these juveniles (see maican endemic Sesarminae for which the breeding behavior is known. So far, these Table 4. Number of ovigerous females, brood-shells two species are the only land-breeding with megalopae, and shells with juveniles <3-mm CW brachyuran crabs for which a detailed ac- found in Windsor, Jamaica, from March-December count of the breeding behavior and juvenile 1991. The search activities for shells differed within months, and brood-shells were only occasionally development in the field is available. Al- searched for in August and December, n = no obser- vations. -

Notes and Bibliography on the Larvae of Xanthid Crabs, with a Key to the Known Xanthid Zoeas of the Western Atlantic and Gulf of Mexico
NOTES AND BIBLIOGRAPHY ON THE LARVAE OF XANTHID CRABS, WITH A KEY TO THE KNOWN XANTHID ZOEAS OF THE WESTERN ATLANTIC AND GULF OF MEXICO JOEL W. MARTIN Made in United States of America Reprinted from BULLETIN OF MARINE SCIENCE Vol, 34, No. 2, March 1984 1984 by the Rosenstiel School of Marine and Atmospheric Science of the University of Miami BULLETIN OF MARINE SCIENCE, 34(2): 220-239, 1984 NOTES AND BIBLIOGRAPHY ON THE LARVAE OF XANTHID CRABS, WITH A KEY TO THE KNOWN XANTHID ZOEAS' OF THE WESTERN ATLANTIC AND GULF OF MEXICO Joel W. Martin ABSTRACT The known xanthid crab zoeas can be assigned to six groups, based primarily upon mor phology of the antennal exopod. A brief description of each group is given, and a table listing all known zoeas in each group is presented. The abbreviated number of zoeal stages in some xanthid species seems not attributable solely to restricted environments; however, no alter native reason for abbreviated development in xanthids is known. A key is given for identi fication of 22 xanthid zoeas in the western Atlantic and Gulf of Mexico for which descriptions are available, and a bibliography of all known descriptions of xanthid larvae is included. The first published mention of a larval stage belonging to the brachyuran family Xanthidae MacLeay, 1838, is a short communication by J. Vaughn Thompson (1836). In this paper, Thompson noted that the larval stages of the genus Eriphia Latreille, 1817 and other brachyuran genera corresponded to the genus Zoea of earlier workers. Since that time, the larvae of crabs of the family Xanthidae {sensu lato, not sensu Guinot, 1978) have received a considerable amount of attention. -

Behavioural and Molecular Evidence for the Systematic Position of Macrophthalmus (Hemiplax) Hirtipes Hombron & Jacquinot, 18
BEHAVIOURAL AND MOLECULAR EVIDENCE FOR THE SYSTEMATIC POSITION OF MACROPHTHALMUS (HEMIPLAX) HIRTIPES HOMBRON & JACQUINOT, 1846, WITH COMMENTS ON MACROPHTHALMINE SUBGENERA (DECAPODA, BRACHYURA, MACROPHTHALMIDAE) BY COLIN L. MCLAY1,3), JUN KITAURA2) and KEIJI WADA2) 1) School of Biological Sciences, Canterbury University, Christchurch, 8004, New Zealand 2) Department of Biological Science, Faculty of Science, Nara Women’s University, Kitauoya-nishimachi, Nara 630-8506, Japan ABSTRACT The analysis of 16s rRNA data from the New Zealand sentinel crab, Macrophthalmus (Hemiplax) hirtipes (Jacquinot & Hombron, 1846) (Macrophthalminae: Macrophthalmidae) shows that it is the most basal species in the genus Macrophthalmus, and behavioural trait mapping suggests that ancestral behaviours included extended cheliped fighting. Other behaviour found in some sentinel crabs, such as allocleaning and waving displays, were absent in the ancestor and are regarded here as derived traits. It is proposed that the two subgenera Hemiplax and Tasmanoplax be elevated to full generic status. Three subgenera appear to be polyphyletic: Macrophthalmus (26 species), Mareotis (14 species) and Paramareotis (4 species), and to resolve this problem molecular data from the remaining species will need to be added into the analysis. The Australasian fauna contains the four most basal genera of the Macrophthalminae: Tasmanoplax, Hemiplax, Australoplax,andEnigmaplax. Our hypothesis is that these were early off-shoots from Tethyan ancestor(s) that colonized Australasia. RÉSUMÉ L’analyse des données d’ARNr 16S du crabe sentinelle venant de la néo-zélandais, Macroph- thalmus (Hemiplax) hirtipes (Jacquinot et Hombron, 1846) (Macrophthalminae: Macrophthal- midae) montre que cette espèce est la plus basale du genre Macrophthalmus, et l’analyse des traits comportementaux suggère que le comportements de ses les ancêtres incluaient des com- bats avec les chélipédes déployés. -

Decapoda (Crustacea) of the Gulf of Mexico, with Comments on the Amphionidacea
•59 Decapoda (Crustacea) of the Gulf of Mexico, with Comments on the Amphionidacea Darryl L. Felder, Fernando Álvarez, Joseph W. Goy, and Rafael Lemaitre The decapod crustaceans are primarily marine in terms of abundance and diversity, although they include a variety of well- known freshwater and even some semiterrestrial forms. Some species move between marine and freshwater environments, and large populations thrive in oligohaline estuaries of the Gulf of Mexico (GMx). Yet the group also ranges in abundance onto continental shelves, slopes, and even the deepest basin floors in this and other ocean envi- ronments. Especially diverse are the decapod crustacean assemblages of tropical shallow waters, including those of seagrass beds, shell or rubble substrates, and hard sub- strates such as coral reefs. They may live burrowed within varied substrates, wander over the surfaces, or live in some Decapoda. After Faxon 1895. special association with diverse bottom features and host biota. Yet others specialize in exploiting the water column ment in the closely related order Euphausiacea, treated in a itself. Commonly known as the shrimps, hermit crabs, separate chapter of this volume, in which the overall body mole crabs, porcelain crabs, squat lobsters, mud shrimps, plan is otherwise also very shrimplike and all 8 pairs of lobsters, crayfish, and true crabs, this group encompasses thoracic legs are pretty much alike in general shape. It also a number of familiar large or commercially important differs from a peculiar arrangement in the monospecific species, though these are markedly outnumbered by small order Amphionidacea, in which an expanded, semimem- cryptic forms. branous carapace extends to totally enclose the compara- The name “deca- poda” (= 10 legs) originates from the tively small thoracic legs, but one of several features sepa- usually conspicuously differentiated posteriormost 5 pairs rating this group from decapods (Williamson 1973). -

Decapoda, Brachyura, Xanthidae) Obtained in the Laboratory
Gulf and Caribbean Research Volume 13 Issue 1 January 2001 Morphology of the First Zoeal Stage of Platypodiella spectabilis (Herbst, 1794) (Decapoda, Brachyura, Xanthidae) Obtained in the Laboratory Adilson Fransozo UNESP, Brazil Maria Lucia Negreiros-Fransozo UNESP, Brazil Joel W. Martin Natural History Museum of Los Angeles County Sandra E. Trautwein University of California, Los Angeles Follow this and additional works at: https://aquila.usm.edu/gcr Part of the Marine Biology Commons Recommended Citation Fransozo, A., M. L. Negreiros-Fransozo, J. W. Martin and S. E. Trautwein. 2001. Morphology of the First Zoeal Stage of Platypodiella spectabilis (Herbst, 1794) (Decapoda, Brachyura, Xanthidae) Obtained in the Laboratory. Gulf and Caribbean Research 13 (1): 71-77. Retrieved from https://aquila.usm.edu/gcr/vol13/iss1/8 DOI: https://doi.org/10.18785/gcr.1301.08 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf and Caribbean Research by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Gulf and Caribbean Research Vol. 13, 71–77, 2001 Manuscript received October 6, 2000; accepted January 5, 2001 MORPHOLOGY OF THE FIRST ZOEAL STAGE OF PLATYPODIELLA SPECTABILIS (HERBST, 1794) (DECAPODA, BRACHYURA, XANTHIDAE) OBTAINED IN THE LABORATORY Adilson Fransozo1, Maria Lucia Negreiros-Fransozo1, Joel W. Martin2, and Sandra E. Trautwein2, 3 1Departamento de Zoologia, IB, UNESP, 18618-000, Botucatu ( SP), Brazil, NEBECC (Group of Studies on Crustacean Biology, Ecology and Culture), E-mail: [email protected] and [email protected] 2Natural History Museum of Los Angeles County, Los Angeles, California 90007, USA 3University of California Los Angeles Abstract Ovigerous females of the xanthid crab Platypodiella spectabilis (Herbst, 1794) were obtained from 2 widely separated localities: the Ubatuba coast (Félix Beach, São Paulo) of Brazil and Guana Island in the British Virgin Islands (BVI). -

Hermit Crab Population Ecology on a Shallow Coral Reef (Bailey’S Cay, Roatan, Honduras): Octopus Predation and Hermit Crab Shell Use
Memoirs of Museum Victoria 60(1): 35–44 (2003) ISSN 1447-2546 (Print) 1447-2554 (On-line) http://www.museum.vic.gov.au/memoirs Hermit crab population ecology on a shallow coral reef (Bailey’s Cay, Roatan, Honduras): octopus predation and hermit crab shell use SANDRA L. GILCHRIST New College of Florida, 5700 N. Tamiami Trail, Sarasota, Florida 34243, USA ([email protected]) Abstract Gilchrist, S.L. 2003. Hermit crab population ecology on a shallow coral reef (Bailey’s Cay, Roatan, Honduras): octopus predation and hermit crab shell use. In: Lemaitre, R., and Tudge, C.C. (eds), Biology of the Anomura. Proceedings of a symposium at the Fifth International Crustacean Congress, Melbourne, Australia, 9–13 July 2001. Memoirs of Museum Victoria 60(1): 35–44. Shells can be a limiting factor in allowing hermit crab populations to increase. Predators of gastropod molluscs and of hermit crabs release shells into reef environments where hermit crabs find and cycle them within their populations. Predators also play a role in distributing shells among hermit crab species. To highlight how octopuses influence shell availability to hermit crabs, observations were made on members of Octopus vulgaris Cuvier, 1797 and O. briareus Robson, 1929 at Bailey’s Cay Reef (Roatan, Honduras) during July and August each of three years, 1999–2001. In addi- tion to feeding while foraging, Octopus vulgaris and O. briareus individuals create shell and debris middens outside of their temporary dens. These middens concentrate shells and food for hermit crabs in the reef environment where locat- ing an empty shell could be difficult. -

Number of Ghost Crab Burrows Does Not Correspond to Population Size
Cent. Eur. J. Biol. • 8(9) • 2013 • 843-847 DOI: 10.2478/s11535-013-0208-7 Central European Journal of Biology Number of ghost crab burrows does not correspond to population size Communication Willian T.A.F. Silva1,2,*, Tereza C.S. Calado1 1Integrated Laboratories of Marine and Natural Sciences, Federal University of Alagoas, 57051-090 Maceió-AL, Brazil 2Institute of Evolutionary Sciences of Montpellier, Group of Developmental and Evolutionary Biology, University of Montpellier 2, 34095 Montpellier, France Received 12 December 2012; Accepted 08 May 2013 Abstract: Ghost crabs are distributed worldwide on sandy beaches, and several studies have associated the number of ghost crab burrows with the levels of anthropogenic impacts on the beaches under study. However, our results show that the use of ghost crab Ocypode quadrata burrows to assess levels of anthropogenic impacts on sand beaches may not be accurate, as previously thought, because the number of burrows does not represent an estimate of the population size. In addition, we propose three hypotheses to explain the extremely low number of individuals/number of burrows ratio: the “secret chamber”, the “multiple openings”, and the “one crab, several burrows” hypotheses. We also observed an unusual sex ratio. Keywords: Ocypode quadrata • Ocypodidae • Sandy beaches • Anthropogenic impacts © Versita Sp. z o.o. 1. Introduction [12-15]. In a field survey, we tested the hypothesis that the number of ghost crab O. quadrata burrows on sandy The ghost crab Ocypode quadrata (Fabricius, 1787), beaches corresponds to the estimated number of crabs also known as maria-farinha in Brazil, is the only in the population.