Crystal Structure of Sodium Ethoxide (C2h5ona), Unravelled After a 180 Years
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

(12) Patent Application Publication (10) Pub. No.: US 2005/0044778A1 Orr (43) Pub
US 20050044778A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0044778A1 Orr (43) Pub. Date: Mar. 3, 2005 (54) FUEL COMPOSITIONS EMPLOYING Publication Classification CATALYST COMBUSTION STRUCTURE (51) Int. CI.' ........ C10L 1/28; C1OL 1/24; C1OL 1/18; (76) Inventor: William C. Orr, Denver, CO (US) C1OL 1/12; C1OL 1/26 Correspondence Address: (52) U.S. Cl. ................. 44/320; 44/435; 44/378; 44/388; HOGAN & HARTSON LLP 44/385; 44/444; 44/443 ONE TABOR CENTER, SUITE 1500 1200 SEVENTEENTH ST DENVER, CO 80202 (US) (57) ABSTRACT (21) Appl. No.: 10/722,127 Metallic vapor phase fuel compositions relating to a broad (22) Filed: Nov. 24, 2003 Spectrum of pollution reducing, improved combustion per Related U.S. Application Data formance, and enhanced Stability fuel compositions for use in jet, aviation, turbine, diesel, gasoline, and other combus (63) Continuation-in-part of application No. 08/986,891, tion applications include co-combustion agents preferably filed on Dec. 8, 1997, now Pat. No. 6,652,608. including trimethoxymethylsilane. Patent Application Publication Mar. 3, 2005 US 2005/0044778A1 FIGURE 1 CALCULATING BUNSEN BURNER LAMINAR FLAME VELOCITY (LFV) OR BURNING VELOCITY (BV) CONVENTIONAL FLAME LUMINOUS FLAME Method For Calculating Bunsen Burner Laminar Flame Velocity (LHV) or Burning Velocity Requires Inside Laminar Cone Angle (0) and The Gas Velocity (Vg). LFV = A, SIN 2 x VG US 2005/0044778A1 Mar. 3, 2005 FUEL COMPOSITIONS EMPLOYING CATALYST Chart of Elements (CAS version), and mixture, wherein said COMBUSTION STRUCTURE element or derivative compound, is combustible, and option 0001) The present invention is a CIP of my U.S. -

SOM KINETIC STUDIES OH KETONE FORMATION THESIS Submitted for the Degree of Doctor of Philosophy at Glasgow University Hy Margare
S O M KINETIC STUDIES OH KETONE FORMATION THESIS submitted for the Degree of Doctor of Philosophy at Glasgow University hy Margaret Bennett Thornley May, 1956 Supervisor Dr. R. I. Reed. ProQuest Number: 13848948 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 13848948 Published by ProQuest LLC(2019). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 4 8 1 0 6 - 1346 TABLE OF CONTENTS. Page. SUMMARY. 2 Part I. HISTORICAL INTRODUCTION. 5 EXPERIMENTAL RESULTS. 13 Comparison of Reaction Rates in Different Media. 8® THEORETICAL INTERPRETATION OP RESULTS. S3 KINETIC OBSERVATIONS. 38 Dilatometry. 35 Colorimetric Estimation. 39 Chemical Analysis. 40 Experimental Determination. 43 Specimen Results. 44 Dieckmann Ring Closure of Diethyl Pimelate. 46 FORMAL PROOF OF UNIQUE RING FORMATION. 50 Experimental Procedure. 53 PREPARATIVE WORK. 55 Part II. HISTORICAL SURVEY. 59 PRELIMINARY EXPERIMENTAL WORK. 69 Preparation and Analysis of Sodium Salts. 71 KINETIC OBSERVATIONS. 74 Experimental Method. 75 Specimen Results. 76 EXPERIMENTAL RESULTS. 81 Page. EXPERIMENTAL RESULTS. 81 Mathematical Analysis. 82 Effect of Nature of Reaction Vessel. 86 To Investigate the Effect of Reaction Products. 89 Pyrolysis of Disodium (3-methyladipate. -

Art-Of-Drugs-Synthesis.Pdf
THE ART OF DRUG SYNTHESIS THE ART OF DRUG SYNTHESIS Edited by Douglas S. Johnson Jie Jack Li Pfizer Global Research and Development Copyright # 2007 by John Wiley & Sons, Inc. All rights reserved. Published by John Wiley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning, or otherwise, except as permitted under Section 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, Inc., 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 750-4470, or on the web at www.copyright.com. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at http://www.wiley.com/go/permission. Limit of Liability/Disclaimer of Warranty: While the publisher and author have used their best efforts in preparing this book, they make no representations or warranties with respect to the accuracy or completeness of the contents of this book and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives or written sales materials. The advice and strategies contained herein may not be suitable for your situation. You should consult with a professional where appropriate. -

REARRANGEMENTS of ALLENES and ACETYLENES by MICHAEL
REARRANGEMENTS OF ALLENES AND ACETYLENES by MICHAEL McPHERSON Thesis submitted for the degree of Doctor of Philosophy University of Edinburgh 1983 I ,•,1. tTAI Acknowledgements I would like to thank Dr. I.H. Sadler for his assistance and encouragement, and the Staff and Technical Staff of the Chemistry Department for their assistance throughout the period of this work. I also wish to thank my parents for their support and encouragement. Finally I wish to thank Mrs. C.G. Ranken for her excellent typing of this thesis. Abstract of Thesis This thesis describes the preparation of alkynyl derivatives of fluorene via reaction of the fluorenyl anion (generated with phenyl lithium) with alkynyl halides. Low pressure vapour phase pyrolysis of these compounds was carried out. Propargylic fluorenes gave allenyl fluorenes by an intramolecular sigmatropic shift with other, radical derived, products predominating at higher temperatures.. The alkynyl fluorenes were themselves resistant to rearrangement by base but the allenyl fluorenes rearranged readily on alumina to give dibenzofulvenes. A number of alkynyl derivatives of indene were prepared by first generating the indenyl anion with sodamide in liquid ammonia or by aqueous alkali under phase transfer catalysis conditions. The anion thus generated was then. reacted with alkynyl halides. Propargylic indenes gave allenyl indenes on pyrolysis and it is shown that the allenes are produced by an intramolecular sigmatropic. shift or a 'Cope' rearrangement depending on the nature of the alkynyl indene. The alkynyl indenes rearrange by base to give benzofulvenes. The benzo- fulvenes were produced as mixtures of geometric isomers some of which were separable. The individual isomers or mixtures of isomers were characterised largely by use of high field 'H n.m.r. -

'({JIXOAA* Laiuoaim ' ' " Adviser Department of Chemistry DEDICATION
SYNTHESIS AND REACTIONS OF INTERMEDIATES FOR STEROID ELABORATION DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Arlen B. Mekler, B.S., M.S. • • • o o The Ohio State University 1959 Approved by '({JIXOAA* lAiUOAim ' ' " Adviser Department of Chemistry DEDICATION TO LEV A. MEKLER ii ACKNOWLEDGMENT The author wishes to express his sincere appreciation to Dr. Melvin S. Newman for stimulating ideas and helpful advice, for interesting discussions and constructive criticisms, for invaluable laboratory techniques, for his personal friendship and his inspirational guidance throughout this research. The author is also grateful for the receipt of the following awards: Charles P. Kettering Founda tion Fellowship, 1956-1957; Melvin S. Newman Scholarship, 1957; U. S. Industrial Company Fellow ship, 1957-1958; Allied Chemical Company Fellowship, 1958-1959; and E. I. Du Pont Research Fellowship, 1959. iii TABLE OP CONTENTS Page ACKNOWLEDGMENT. ... .. ill OBJECTIVE 1 HISTORICAL BACKGROUND 4 DISCUSSION OP RESULTS 20 SUGGESTIONS FOR FURTHER WORK 39 EXPERIMENTAL «,.... 42 General Procedures, 42 2-Methyl-l, 3-cyclohexanedione 43 4-Diethylamino-3-butanone0 44 1,6-Dioxo-8a-methyl-l, 2, 3, 4, 6,7,8, 8a- oc tahydronaphthalene. ••• 44 K -(6-Methyl-3-keto-l-cyclohexene- 1-yl)-butyric acid 47 Methyl ^-(6-Methyl-3-keto-l-cyclo- hexene-1-yl) butyrate • 48 Methyl &"-(3-Acetoxy-6-methyl-l,3- cyclohexadien-1-yl) butyrate. 49 Methyl #-( 5-Hydroxy-2-methylphenyl) - butyrate 50 Action of Sodium Methoxide on Methyl ^-{ 5-Hydroxy-2-methylphenyl) butyrate 51 3- (4-Hydroxybutyl) - 4-me thylphenol 51 3- ( 4-Bromobutyl) - 4-me thylphenol • 52 Attempted Synthesis of 3-(4-bromobutyl)- 4-Methylphenol Employing Acetic Acid. -

Tin Ethoxide and Related Compounds
INIS-SD--161 SD0100019 TIN ETHOXIDE AND RELATED COMPOUNDS A DISSERTATION TO FULIFIL THE REQUIREMENTS OF MASTER DEGREE OF SCIENCE(M.Sc) Submitted By: AZZA IZZELDIN MOHAMED OMER Supervisor : Prof. AHMED KHIDIR YAGOUB Co-Supervisor: Dr. OMER YO&IF OMER UNIVERSITY OF KHARTOUM FACULTY OF EDUCATION DEPARTMENT OF CHEMISTRY JUNE 2000 Content: Section One Historical Chapter One Some Aspects of Tin Chemistry Introduction : (1-1) Elemental Tin. (1-2)Bonds Forming. (1-3) The Nature of (Sn-O)Bond . (1-4) Synthesis of Tin Alkoxides. (l-4-l)The Direct Route. (l-4-2)The Reaction between Tetrachlorosilane and Alcohol. (l-4-3)The Reaction between Tintetrachloide and Sodium Ethoxide. (l-4-4)Other Methods. (1-5) Double Alkoxides of Tin. (1-6) Uses of Metal Alkoxides . Chapter Two Some Selected Physical & Chemical Properties of Tin Alkoxides . (2-1) Physical Properties of Tin Alkoxides . (2-2) Infra-Red Spectrum of Tin Alkoxides . (2-3)Gas-Liquid Chromatography of Alkoxides . (2-4)Some Selected Chemical Properties of Tin Alkoxides. (2-4-1) Hydrolysis Reaction. (2-4-2) Reaction with Halogens, Hydrogen Halides & Acyl Halides . (2-4-3) Reaction with Alcohols . Section Two Experimental Chapter Three Experimental (3) Experimental. (3-1) General Techniques & Procedures . (3-1-1) Infra-Red Spectrum. (3-l-2)Gas-Liquid Chromatography. (3-2) Starting Materials. (3-2-l)Ethanol. (3-2-2)Tin Foil. (3-2-3)Tin Tetra Chloride. (3-2-4)Sodium Metal. (3-2-5)Mercury(I) Chloride. (3-3)Reaction Procedure & Apparatus . (3-3-1 )The Reaction between Tin Foil & Dry Ethanol Using Mercury(I) Chloride Catalyst. -

Safety Data Sheet
SAFETY DATA SHEET Creation Date 09-Sep-2009 Revision Date 27-Dec-2020 Revision Number 2 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product Description: Sodium ethoxide, 21% w/w in ethanol Cat No. : L13083 Molecular Formula C2 H5 Na O Unique Formula Identifier (UFI) 4U9Q-YTY1-1W06-UQDY 1.2. Relevant identified uses of the substance or mixture and uses advised against Recommended Use Laboratory chemicals. Uses advised against No Information available 1.3. Details of the supplier of the safety data sheet Company Alfa Aesar . Avocado Research Chemicals, Ltd. Shore Road Port of Heysham Industrial Park Heysham, Lancashire LA3 2XY United Kingdom Office Tel: +44 (0) 1524 850506 Office Fax: +44 (0) 1524 850608 E-mail address [email protected] www.alfa.com Product Safety Department 1.4. Emergency telephone number Call Carechem 24 at +44 (0) 1865 407333 (English only); +44 (0) 1235 239670 (Multi-language) Poison Centre - Emergency Ireland : National Poisons Information Centre (NPIC) - information services 01 809 2166 (8am-10pm, 7 days a week) Malta : +356 2395 2000 Cyprus : +357 2240 5611 SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the substance or mixture CLP Classification - Regulation (EC) No 1272/2008 Physical hazards Flammable liquids Category 3 (H226) ______________________________________________________________________________________________ ALFAAL13083 Page 1 / 12 SAFETY DATA SHEET Sodium ethoxide, 21% w/w in ethanol Revision Date 27-Dec-2020 ______________________________________________________________________________________________ Health hazards Skin Corrosion/Irritation Category 1 B (H314) Serious Eye Damage/Eye Irritation Category 1 (H318) Environmental hazards Based on available data, the classification criteria are not met Full text of Hazard Statements: see section 16 2.2. -

Synthesis and Characterization of Sodium Alkoxides
Bull. Mater. Sci., Vol. 29, No. 2, April 2006, pp. 173–179. © Indian Academy of Sciences. Synthesis and characterization of sodium alkoxides K CHANDRAN, R NITHYA†, K SANKARAN, A GOPALAN‡ and V GANESAN* Materials Chemistry Division, †Materials Science Division, Indira Gandhi Centre for Atomic Research, Kalpakkam 603 102, India ‡Department of Industrial Chemistry, Alagappa University, Karaikudi 630 006, India MS received 21 April 2005; revised 4 February 2006 Abstract. Alcohol route is being adopted for cleaning sodium from sodium-wetted small components of coolant circuits of fast reactors. For better understanding of sodium–alcohol reactions and their energetics, the data on thermo-chemical properties such as heats of sodium–alcohol reactions, heats of dissolution, heat capacities, thermal decomposition behaviour, etc of their end products are essential. In order to generate such data, high purity sodium alkoxides, viz. sodium methoxide, sodium ethoxide and sodium n-propoxide, were prepared by reacting sodium metal with respective alcohol. These compounds were characterized using X-ray diffraction technique and IR spectroscopy. The elemental analysis was carried out by CHNS analyser and atomic emis- sion spectroscopy. Normal chain sodium alkoxides were found to exhibit tetragonal crystal structure. Crystal structures of sodium ethoxide and sodium n-propoxide are reported for the first time. The IR spectrum of sodium n-propoxide is also reported for the first time. Keywords. Sodium methoxide; sodium ethoxide; sodium n-propoxide; IR spectra; X-ray diffraction; crystal structure. 1. Introduction Low molecular weight alcohols viz. methanol and ethanol, are generally employed for cleaning sodium-wetted small Favourable physical, chemical and nuclear properties as components such as valves, bellows, etc. -
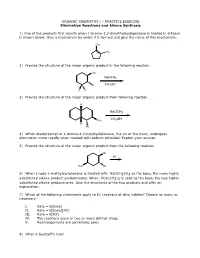
ORGANIC CHEMISTRY I – PRACTICE EXERCISE Elimination Reactions and Alkene Synthesis 1) One of the Products That Results When 1
ORGANIC CHEMISTRY I – PRACTICE EXERCISE Elimination Reactions and Alkene Synthesis 1) One of the products that results when 1-bromo-2,2-dimethylcyclopentane is heated in ethanol is shown below. Give a mechanism by which it is formed and give the name of this mechanism. CH3 CH3 2) Provide the structure of the major organic product in the following reaction. CH3 NaOCH3 Br CH3OH H D 3) Provide the structure of the major organic product from following reaction. H NaOCH3 CH3OH CH3 H Br 4) Which diastereomer of 1-bromo-4-t-butylcyclohexane, the cis or the trans, undergoes elimination more rapidly when treated with sodium ethoxide? Explain your answer. 5) Provide the structure of the major organic product from the following reaction. Br KI H3C Br 6) When 1-iodo-1-methylcyclohexane is treated with NaOCH2CH3 as the base, the more highly substituted alkene product predominates. When KOC(CH3)3 is used as the base, the less highly substituted alkene predominates. Give the structures of the two products and offer an explanation. 7) Which of the following statements apply to E1 reactions of alkyl halides? Choose as many as necessary. I. Rate = k[base] II. Rate = k[base][RX] III. Rate = k[RX] IV. The reactions occur in two or more distinct steps. V. Rearrangements are sometimes seen. 8) What is Saytzeff's rule? 9) What major product results when 2-bromo-2-methylbutane is treated with sodium ethoxide. 10) How many distinct alkenes can result from E2 elimination of the compound below? Give their structures and IUPAC names. -

Substituted Pyridine and Quinoline Sulfides Mary Alys Plunkett Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1947 Substituted pyridine and quinoline sulfides Mary Alys Plunkett Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Plunkett, Mary Alys, "Substituted pyridine and quinoline sulfides" (1947). Retrospective Theses and Dissertations. 15128. https://lib.dr.iastate.edu/rtd/15128 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overiaps. -

Candesartan Cilexetil
Tetrahedron 66 (2010) 5402e5406 Contents lists available at ScienceDirect Tetrahedron journal homepage: www.elsevier.com/locate/tet A novel and practical synthesis of substituted 2-ethoxy benzimidazole: candesartan cilexetil Ping Wang a, Guo-jun Zheng b, Ya-ping Wang b, Xiang-jing Wang a, Yan Li a, Wen-sheng Xiang a,* a Department of Biochemical Engineering, Northeast Agricultural University, Harbin 150030, China b Zhejiang Hisun Pharmaceutical Co., Ltd., Taizhou 318000, China article info abstract Article history: A novel and practical synthetic route for the preparation of candesartan cilexetil from methyl 2-amino-3- Received 16 January 2010 nitrobenzoate is described. The key steps are the reaction of methyl 2-bromo-3-(diethoxy- Received in revised form 26 April 2010 methyleneamino)benzoate with (20-(1-trityl-1H-tetrazol-5-yl) biphenyl-4-yl) methanamine and the final Accepted 11 May 2010 formation of 2-ethoxy benzimidazole ring via intramolecular N-arylation. The final ring closure process Available online 31 May 2010 could be utilized to prepare other 2-substituted benzimidazoles. The method is simple for operation and suitable for industrial production. Keywords: Ó 2010 Elsevier Ltd. All rights reserved. Candesartan cilexetil Receptor antagonist Antihypertensive Synthesis 1. Introduction In recent years, extensive efforts have been drafted toward the convenient and efficient method for the synthesis of candesartan e The development of angiotensin receptor blockers (ARBs) is cilexetil 1 and several methods have been reported.12 19 In general, e a major advance for the treatment of hypertension and potentially these methods include three routes (Scheme 1): In route A12 15, for other cardiovascular disorders.1 Candesartan, 2-ethoxy-1-[[20- compound 4 was applied as the key intermediate.