Phytochemical Analysis, Antimicrobial and Antioxidant Activity Evaluations of Fruit of Artocarpus Heterophyllus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Role of Co-Operative Societies in Black Clam Fishery and Trade in Vembanad Lake
6 Marine Fisheries Information Service T&E Ser., No. 207, 2011 Role of co-operative societies in black clam fishery and trade in Vembanad Lake N. Suja and K. S. Mohamed Central Marine Fisheries Research Institute, Kochi Lime shells and live clams are distributed in large quantities in the backwaters and estuaries of Kerala. Vembanad, the largest lake of Kerala, also holds a vast resource of lime shells and live clam, comprising several species. The major species that account for the clam fishery of Vembanad Lake is the black clam Villorita cyprinoides. The lime shells that contribute to the fishery are broadly classified as the ‘white shells’ and the ‘black shells’. The so-called ‘white shells’ are sub-soil deposits of fossilized shells and are known to extend upto 7 feet below the lake bottom. The black shells are obtained from the living population of V. cyprinoides, which contribute more than 90% of the clams from this lake. The lime shell is mainly used for the manufacture of cement, calcium carbide and sand lime bricks. They are also used for lime burning, for construction, in paddy field / fish farms for neutralizing acid soil and as slaked lime. This is used as a raw material for the manufacture of distemper, glass, rayon, paper and sugar. Shell Control Act The Government of India has listed lime shell as a minor mineral under the Mineral Concession Fig. 1. Location of black clam lime shell industrial co-operative Rules, 1949, Section 5 of the Mines and Minerals. societies The acquisition, sale, supply and distribution of lime shell in the State are at present controlled by Black clam lime shell industrial co-operative the Kerala Lime Shells Control Act, 1958. -

Preliminary Minutes of the 5Th Meeting of the Syndicate Held on 19.02.2019
1 UNIVERSITY OF KERALA (Re-accredited by NAAC with ‘A’ Grade) Preliminary Minutes of the 5th Meeting of the Syndicate held on 19.02.2019 Place of Meeting : University Buildings Thiruvananthapuram Time : 12.00 Noon Members present: 1. Prof.(Dr.) V.P.Mahadevan Pillai (In the Chair) Vice–Chancellor 2. Prof.(Dr.) P.P.Ajayakumar Pro-Vice-Chancellor 3. Adv.K.H.Babujan 4. Adv.G.Sugunan 5. Sri.M.Sreekumar 6. Dr.K.B.Manoj 7. Dr.Shaji.K 8. Sri.B.Unnikrishnan Nair 9. Dr.P.Rajesh Kumar 10. Dr.S.Nazeeb 11. Sri.Shijukhan J.S 12. Sri.M.Harikrishnan 13. Sri.M.Lenin Lal 14. Dr.K.R.Kavitha Item No.05.01. Confirmation of the Preliminary Minutes of the 3 rd Meeting of the Syndicate held on 01.02.2019 - reg. (Ac.A.I) The Syndicate considered and approved the Preliminary Minutes of the 3 rd Meeting of the Syndicate held on 01.02.2019. ======================================================================= Item No.05.02. Confirmation of the Preliminary Minutes of the 4 th Meeting of the Syndicate held on 08.02.2019 - reg. (Ac.A.I) The Syndicate considered and approved the Preliminary Minutes of the 4th Meeting of the Syndicate held on 08.02.2019 with the following modification: Item No.04.08.01 Resolution be modified as ‘ RESOLVED that the above recommendations of the combined meeting of the Standing Committees of the Syndicate on Examinations and Standing Committee of the Syndicate on Finance, held on 28.01.2019, be approved and FURTHER RESOLVED to authorize the Director, Computer Centre and Dr.Aji.S, Head, Department of Computer Science to coordinate with the counterparts in MG University regarding sharing of examination software for optimum use at lesser cost, and also to ensure data safety by additional alternate storage ’ (M&C) ======================================================================= 2 Item No.05.03. -

Alappuzha Alleppey the Heart of Backwaters
Alappuzha Alleppey The Heart of Backwaters STD Code +91 477 Major Railway Stations Alappuzha Cherthala Chengannur Mavilikkara Kayamkulam Closest Airport Cochin International Airport 7 The wind slowly wafts through the rolling paddy fields, swaying palm fronds to the vast, sedate backwaters. Life has a slow pace in the almost magical village life of Alappuzha. The greenery that stretches as far as eyes can reach, the winding canals, enthralling backwaters, pristine nature makes Alappuzha a dream come true for the casual and serious traveller. The name Alappuzha is derived from Aal Even from the early periods of celebrated historic importance of Alappuzha District. (Sea)+ puzhai (River-mouth). The district ‘Sangam’ age, Kuttanad, known as the Christianity had a foothold in this of Alappuzha (Aleppey) was formed in the rice bowl of Kerala, with its paddy fields, district, even from the 1st century AD. The 17th August, 1957, carving regions out of small streams and canals with lush green church located at Kokkamangalam was the erstwhile Quilon (Kollam) and Kotta- coconut palms, was well known. The one of the seven churches founded by St. yam districts, spreading in 1414sq.km. The name Kuttanad is ascribed to the early Thomas, one of the twelve disciples of district headquarters is at Alappuzha. Cheras who were called the Kuttavans. Jesus Christ. It is generally believed that Alappuzha, the backwater heartland dis- Literary works like “Unnuneeli Sandesam”, he landed at Maliankara in Muziris Port, trict of Kerala, exudes all the bewitching one of the oldest literary works of Kerala, later came to be known as Cranganore charm that Kerala has. -

Cherthala School Code Sub District Name of School School Type 34337 Thuravoor GUPS Thycattussery G 34009 Thuravoor HSS Kandamang
Cherthala School Code Sub District Name of School School Type 34337 Thuravoor GUPS Thycattussery G 34009 Thuravoor HSS Kandamangalam A 34330 Thuravoor GLPS Thaliyaparambu G 34331 Thuravoor GLPS Mattathil Bhagam G 34332 Thuravoor St. Francis Xaviers LPS Eramalloor A 34333 Thuravoor BBM LPS Azheekal A 34334 Thuravoor GUPS Arookutty G 34328 Thuravoor St. Marys LPS Srambickal A 34336 Thuravoor GUPS Uzhuva G 34327 Thuravoor NSS LPS Panavally A 34338 Thuravoor GUPS Kadakkarappally G 34339 Thuravoor GUPS Odampally G 34340 Thuravoor GUPS Parayakad G 34341 Thuravoor GUPS Changaram G 34342 Thuravoor St. Martins UPS Neendakara A 34004 Thuravoor St. Augustines KSS Aroor A 34343 Thuravoor NI UPS Naduvath Nagar A 34335 Thuravoor GUPS Thuravor West G 34320 Thuravoor SN LPS Ezhupunna A 34007 Cherthala Govt HS Pollathai G 34006 Cherthala Govt HSS Kalavoor G 34003 Cherthala St. Augustines HS Mararikulam A 34002 Cherthala Govt.Fisheries HS Arthungal G 34001 Cherthala SFA HSS Arthunkal A 34318 Thuravoor Govt TD LPS Thuravoor G 34329 Thuravoor St. Mary Immaculate LPS Ezhupunna A 34319 Thuravoor St.Augustines LPS Aroor A 34010 Thuravoor Sr. Georges HS Thankey A 34321 Thuravoor St. Thomas LPS Pallithode A 34322 Thuravoor LFM LPS Manakkodam Pattam A 34323 Thuravoor St. Josephs LPS Ottamassery A 34317 Thuravoor GLPS Konattussery G 34324 Thuravoor St. Josephs LPS Uzhuva A 34325 Thuravoor Thuravoor Panchayath LPS A 34326 Thuravoor MAM LPS Panavally A 34253 Cherthala KPM UPS Muhamma A 34312 Thuravoor LPGS Perumpalam G 34005 Thuravoor Govt HS Aroor G 34305 Thuravoor -
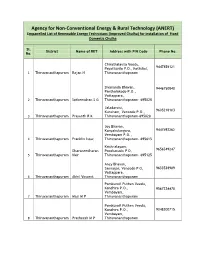
For Installation of Fixed Domestic Chulha
Agency for Non-Conventional Energy & Rural Technology (ANERT) Empanelled List of Renewable Energy Technicians (Improved Chulha) for installation of Fixed Domestic Chulha Sl. District Name of RET Address with PIN Code Phone No. No. Chirathalavila Veedu, 9447858121 Payattuvila P.O., Kottukal, 1 Thiruvananthapuram Rajan N Thiruvananthapuram Sivananda Bhavan, 9446750840 Panthalakode P.O., Vattappara, 2 Thiruvananthapuram Satheendran S G Thiruvananthapuram- 695028 Jaladarsini, 9605218183 Kuncham, Vencode P.O., 3 Thiruvananthapuram Prasanth R K Thiruvananthapuram-695028 Joy Bhavan, Kanyakulangara, 9447492262 Vembayam P.O., 4 Thiruvananthapuram Franklin Issac Thiruvananthapuram- 695615 Krishnalayam, Dharaneedharan Poozhanadu P O, 9656549247 5 Thiruvananthapuram Nair Thiruvananthapuram- 695125 Ancy Bhavan, Sannagar, Vencode P O, 9633538989 Vattappara, 6 Thiruvananthapuram Akhil Vincent Thiruvananthapuram Pomkunnil Puthen Veedu, Konchira P.O., 9567224470 Vembayam, 7 Thiruvananthapuram Masi M P Thiruvananthapuram Pomkunnil Puthen Veedu, Konchira P.O., 9048200715 Vembayam, 8 Thiruvananthapuram Pratheesh M P Thiruvananthapuram Dhanesh Bhavan, Puthenkodur, 9847487304 Vencode P.O., Vembayam, 9 Thiruvananthapuram Dhanesh Kumar S Thiruvananthapuram- 695028 Pomkunnil Puthen Veedu, Konchira P.O., 9961767315 Vembayam, 10 Thiruvananthapuram Shameer A Thiruvananthapuram Vadakkekara Puthen Veedu, Kottukal, 9946315645 Payattuvila P O, 11 Thiruvananthapuram Sisupalan Thiruvananthapuram Aruvikuzhy Charuvilakathu Veedu, 9495629753 Vencode P.O., 12 Thiruvananthapuram -

Accused Persons Arrested in Alappuzha District from 28.06.2015 to 04.07.2015
Accused Persons arrested in Alappuzha district from 28.06.2015 to 04.07.2015 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, Rank which No. Accused Sex of Law Station Accused Arrested Arrest & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 CHIRAMURICKAL VEEDU, 1 GURUMANDIRAM Cr. 775/15, J.NIZAMUDEE POLICE BAIL WARD, 23.06.2015 U/S 118(a) of AMBALAPU N, S I OF RINEESH SURESH 22, M ALAPPUZHA VANDANAM 22.55 KP Act ZHA POLICE LIJUMON Cr. 776/15, 2 POLICE BAIL VARGHEES CHIRAYIL VEEDU, 24.06.2015 U/S 118(a) of AMBALAPU A.M.KABEER, E VARGHESE 30, M PURAKKAD P W-6 PURAKKAD 15.10 KP Act ZHA S I of POLICE PUTHUVAL, Cr. 778/15, A.M.KABEER, 3 POLICE BAIL AMBALAPUZHA P AMBALAPUZ 24.06.2015 U/S 118(a) of AMBALAPU S I of SHAMNAD THAJUDEEN 21, M W-1 HA jn 18.15 KP Act ZHA POLICE PADINJARE NADA, Cr. 779/15, 4 SUBY MANDIRAM, u/s 279 IPC & POLICE BAIL VINICHAND AMBALAPUZHA P AMBALAPUZ 24.06.2015 185 of MV AMBALAPU A.M.KABEER, RAN CHANDRAN 35, M W-6 HA 21.05 Act ZHA S I of POLICE Cr. 780/15, 5 KALATHIL VEEDU, NEAR u/s 279 IPC & POLICE BAIL AMBALAPUZHA RAILWAY 25.06.2015 185 of MV AMBALAPU K.A.PAUL, S DINESAN VENU 40, M NORTH P W-4 GATE 20.55 Act ZHA I of POLICE Cr. -

HPTLC Screening of Amino Acids from Acorus Calamus Rhizome and Ardisia Solanacea Leaf from Kuttanad Wetlands, Kerala, India
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2014, 6(4):958-962 ISSN : 0975-7384 Research Article CODEN(USA) : JCPRC5 HPTLC screening of amino acids from Acorus calamus rhizome and Ardisia solanacea leaf from Kuttanad Wetlands, Kerala, India Pratap Chandran R.a* , Manju S.a, Vysakhi M. V.a, Shaji P. K.b and Achuthan Nair G. c aDepartment of Biotechnology and Research, K. V. M. College of Engineering and Information Technology, K. V. M. College Road, Kokkothamangalam P. O., Cherthala, Alappuzha District, Kerala State, India bEnvironmental Resources Research Centre (ERRC), P.O. Peroorkada, Thiruvananthapuram, Kerala State, India cChair for Sustainable Development, Indira Gandhi National Open University (IGNOU), Regional Centre, Thiruvananthapuram, Kerala State, India _____________________________________________________________________________________________ ABSTRACT Acorus calamus L., a medicinal herb occasionally planted in the home gardens, and Ardisia solanacea Roxb., a wild edible fruit plant, are frequently found in the Kuttanad wetlands of Kerala state. High Performance Thin Layer Chromatography (HPTLC) technique was used to detect and quantify the amino acids present in the rhizome of Acorus calamus and in the leaves of Ardisia solanacea. Methanolic extracts of the test plants were subjected to HPTLC analysis where n-butanol: acetic acid:water (4:1:1) mixture was used in the mobile phase. Four amino acids, glutamic acid, L-cystine, lysine and serine were detected from A. calamus while alanine, glutamic acid and valine were detected from A. solanacea. Key words: amino acid, Acorus calamus , Ardisia solanacea , HPTLC, methanolic extract _____________________________________________________________________________________________ INTRODUCTION Amino acids are the “building blocks of the proteins”, which serve as body builders and play an important role in the metabolism [1]. -

C:\Users\CGO\Desktop\Final Part
Census of India 2011 KERALA PART XII-A SERIES-33 DISTRICT CENSUS HANDBOOK ALAPPUZHA VILLAGE AND TOWN DIRECTORY DIRECTORATE OF CENSUS OPERATIONS KERALA 2 CENSUS OF INDIA 2011 KERALA SERIES-33 PART XII-A DISTRICT CENSUS HANDBOOK Village and Town Directory ALAPPUZHA Directorate of Census Operations, Kerala 3 MOTIF Nehru Trophy Boat Race Subroided with a labyrinth of backwaters, Alappuzha District is the cradle of important boat races like the Nehru Trophy Boat Race at Punnamada, Pulinkunnu Rajiv Gandhi Boat Race, and the Payippad Jalotsavam at Payippad, Thiruvanvandoor, Neerettupuram Boat Race, Champakulam Boat Race, Karuvatta and Thaikottan races. The boat races are mainly conducted at the time of ‘Onam’ festival. The Nehru Trophy Boat Race was instituted by the then Prime Minister Jawaharlal Nehru in the year 1952, on being thrilled by the enchanting beauty of the racing snake shaped boats. Ever since, the race is being conducted at the time of Onam festival on second Saturday of August every year. Various cultural programmes are also conducted along with the race, creating a festive mood in the town. Thousands of tourists from all over the world flock in, to have a glimpse at this spectacular occasion. 4 CONTENTS Pages 1. Foreword 7 2. Preface 9 3. Acknowledgements 11 4. History and scope of the District Census Handbook 13 5. Brief history of the district 15 6. Analytical Note 17 Village and Town Directory 141 Brief Note on Village and Town Directory 7. Section I - Village Directory (a) List of Villages merged in towns and outgrowths -

Monsoon August 2019
RELIEF CAMP DETAILS (UPDATED 14.08.2019 6.00 PM) No. of No. of Adults Date opened Taluk camps Village Camp name families Male Female Children Total 09-08-2019 1 Chengannur JB School, Keezheri 6 6 6 6 18 Keltron Centre, 09-08-2019 1 Ennakkad Ennakkad 38 49 47 30 126 Thayyur Pakalveedu 09-08-2019 1 Ennakkad Centre, Ennakkad 15 20 18 10 48 09-08-2019 1 Mulakkuzha MP UPS, Puthencavu 25 37 43 13 93 Govt High School, 09-08-2019 Chengannur 1 Thiruvanvandoor Thiruvanvandoor 44 40 79 34 153 Hindu UP School, 09-08-2019 1 Thiruvanvandoor Iramallikkara 29 47 47 17 111 10.08.2019 1 Mannar Kunnathur UPS 53 79 89 19 187 10.08.2019 1 Venmony Ikyavedi club 6 9 7 5 21 10.08.2019 1 Venmony Thachappally LPS 46 47 55 41 143 10.08.2019 Chengannur 1 Ennakkad GHSS Bhudhanoor 49 58 53 21 132 10.08.2019 1 Ennakkad Govt. UPS Ennakkad 45 69 71 28 168 10.08.2019 1 Mulakkuzha LPS Piralasseri 33 49 53 27 129 10.08.2019 1 Pandanad Near Homeo Dispensery 19 29 28 21 78 10.08.2019 Chengannur 1 Pandanad JBS Pandanad 4 5 7 9 21 10.08.2019 1 Cheriyanad JBS Cheriyanad 23 20 28 8 56 10.08.2019 1 Cheriyanad JBS Cheruvalloor 12 12 13 3 28 Karayogam UPS 11.08.2019 1 Kurattissery Pavukara 58 65 52 28 145 11.08.2019 1 Venmony Lohiya Memmorial HS 18 17 28 9 54 Eramallikkara Ayyappa 11.08.2019 1 Thiruvanvandoor College 8 13 11 7 31 Chengannur MS MD LPS 11.08.2019 1 Mannar Kuttamperoor 55 83 83 23 189 11.08.2019 1 Pandanad JBS Kezhvenmazhi 6 12 10 5 27 11.08.2019 Chengannur 1 Puliyoor Govt. -

CUSAT Prospectus.Pdf
COCHIN UNIVERSITY OF SCIENCE AND TECHNOLOGY ACADEMIC PROSPECTUS 2015 INDEX CHAPTER 1 PAGE 2 THE UNIVERSITY - A PRELUDE CHAPTER 2 PAGE 4 TEACHING DEPARTMENTS/ SCHOOLS/CENTRES/ RECOGNIZED INSTITUTIONS - AN OVERVIEW OF THE ACADEMIC SCENARIO CHAPTER 3 PAGE 43 ACADEMIC AND STUDENT SERVICES - GLIMPSES CHAPTER 4 PAGE 52 ACADEMIC PROGRAMMES OF THE UNIVERSITY – FOSTERING EXCELLENCE CHAPTER 5 PAGE 63 ELIGIBILITY CRITERIA – A READYRECKONER CHAPTER 6 PAGE 98 ADMISSION PROCEDURE – GENERAL INSTRUCTIONS CHAPTER 7 PAGE 112 INTERNATIONAL CANDIDATES – READ THIS FIRST CHAPTER 8 PAGE 118 CHILDREN OF INDIAN WORKERS IN GULF COUNTRIES – NOTE THIS CHAPTER 9 PAGE 120 CHOOSING TEST SUBJECTS – A QUICK REFERENCE TABLE CHAPTER 10 PAGE 121 RESERVATIONS – JUST & FAIR CHAPTER 11 PAGE 133 SCHEDULE OF FEES – PLAN YOUR EXPENSES CHAPTER 12 PAGE 141 REFUND OF FEES – RULES ARE IMPORTANT CHAPTER 13 PAGE 142 FEE CONCESSIONS – CHECK ELIGIBILITY CHAPTER 14 PAGE 143 HOSTEL FACILITIES - YOU WILL ENJOY IT CHAPTER 15 PAGE 145 CAMPUS DISCIPLINE - INEVITABLE CHAPTER 16 PAGE 146 PARENT TEACHER ASSOCIATION – BRIDGING RELATIONSHIPS CHAPTER 17 PAGE 147 HOW TO REACH CUSAT – THE WAY TO SUCCESS CHAPTER 18 PAGE 148 TEST CENTRES – WE ARE NEAR YOU CHAPTER 19 PAGE 149 COUNSELLING / ADMISSION INTIMATIONS – BE AWARE & ALERT CHAPTER 20 PAGE 149 IMPORTANT DATES - ALWAYS REMEMBER CUSAT, Academic Prospectus 2015 Page 1 Chapter 1 The University – A Prelude COCHIN UNIVERSITY OF SCIENCE AND TECHNOLOGY Cochin University P O Ph: +91-484 – 2575396 Kochi-682 022 Fax: +91-484-2577595 Kerala Email: [email protected] India Website: www.cusat.nic.in , www.cusat.ac.in JUSTICE SHRI. P SATHASIVAM CHANCELLOR THE HONOURABLE GOVERNOR OF KERALA PRO - CHANCELLOR SHRI. -

Alappuzha District
Page 1 Price. Rs. 100/- per copy UNIVESITY OF KERALA Election to the Senate by the member of the Local Authorities-2013-14 (Under Section 17-Elected Members (7) of the Kerala University Act 1974) Electoral Roll of the Members of the Local Authorities- Alappuzha District Alappuzha Muncipality Roll No. candidate mun_wd.no mun_wardname h_no h_name place pin 1 DIVAKARAN.K 1 Kayamkulam TOWN U P S 11/268 Jyothish Bhavanam Eruva East 690571 2 JALEELA SAKEER.A 2 Kayamkulam KOTTUKULANGARA Nambalasseri vadakkethil Kottukulangara 690502 3 RAMLA.P.T 3 Kayamkulam ARAKKAL 260 B Pazhayatheruvil Kayamkulam 690502 4 KULSUMMA.H 4 Kayamkulam MOHIDEENPALLI 311 Kayyalackal veedu Kayamkulam 690502 5 Advt.SHIJI.A 5 Kayamkulam VALIYAPARAMBU 355 A Kochu kottaikkakath tharayil Kayamkulam 690502 6 VALSALA MOHANDAS 6 Kayamkulam MAAVILATHE 16 Chaithram Kayamkulam 690564 7 SREELATHA.A 7 Kayamkulam ERUVA KSHETHRAM Gokulam Kayamkulam 690572 8 ANITHA AYYAPPAN.K 8 Kayamkulam WARE HOUSE WARD 151 Pokkattu Kizhakkethil Kayamkulam 690502 9 KOCHUKUNJU.P.K 9 Kayamkulam MARKET 434 Sheeja Manzil Kayamkulam 690502 10 LATHABAI.K.V 10 Kayamkulam SREE VEEDOBHA 9/221 Chakkalayil kizhakkethil Kayamkulam 690564 11 SUMITHRAN.R 11 Kayamkulam GURUMANDHIRAM 14/8F Thekkinezhath Kayamkulam 690564 12 AMBILI SURESH 12 Kayamkulam ERUVA KIZHAKKU 335 Radheyam Eruva Kizhakku 690572 13 MINI SAMUEL 13 Kayamkulam KAKKANADU Leya Bhavan Kakkanadu 690559 14 ANSARI.A 14 Kayamkulam MADHRASA 378 Koyikuleth Peringala 690559 15 SABEELA LATHEEF 15 Kayamkulam RAILWAY STATION 284 Kalickal puthen veedu Kayamkulam -

Accused Persons Arrested in Alappuzha District from 08.06.2014 to 14.06.2014
Accused Persons arrested in Alappuzha district from 08.06.2014 to 14.06.2014 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, Rank which No. Accused Sex of Law Station Accused Arrested Arrest & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 Venugopala 22/14, KalVishnu Bhavanam, 08/06/14, Cr. 1156/14, U/s Anoop Jose 1 Vishnu Neduvaramcodu Chengannur Nil Pillai Male Neduvaramcodu, Ala 02.10 hrs. 118(a) of KP Act SI of Police Cr. 1159/14, U/s 41/14, Paramukhathu huse, 08/06/14, N. Manoharan 2 Shibu Raveendran Nandavanam Jn 279 IPC & 185 Chengannur Nil Male Thittamel, Chengannur 14.05 hrs. SI T. Unit of MV Act Cr. 1160/14, U/s 45/14, Puthenveettil, Pandanadu 08/06/14, Anoop Jose 3 Prasannan Thankappan Pandanadu 118(A) of |KP Chengannur Nil Male West, Pandanadu 14.15 hrs. SI of Police Act Valyalil house, K. Baiju Kumar, Produced before Mayandi @ 25/14, 08/06/14, Cr. 1157/14, U/s 4 Krishnan Nedungoloma Village, Chengannur Chengannur Inspector of JFMC I, Kumar Male 16.00 hrs. 376,450 IPC Paravoor, Kolam police Chengannur Santhosh Bhavanam, Cr. 1161/14, U/s 35/14, 08/06/14, Anoop Jose 5 Manoj Sivankutty Venmony West, KSRTC Jn 279 IPC & 185 Chengannur Nil Male 18.00 hrs. SI of Police Venmony of MV Act Malayil Kuttiyil house, Cr. 1162/14, U/s 27/14, 08/06/14, Anoop Jose 6 Prasanth Gopala Pillai Cheriyanadu West, Althara Jn 279 IPC & 185 Chengannur Nil Male 18.20 hrs.