Predictions of Radius Bending Strength by Radius Stiffness, Mineral, and Ulna Mechanical Properties
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
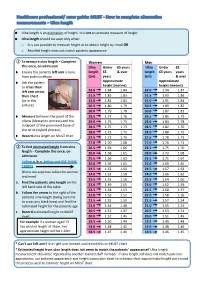
Ulna Length and Mid-Upper
Ulna length is an estimation of height. It is not an accurate measure of height Ulna length should be used only when: o It is not possible to measure height or to obtain height by recall OR o Recalled height does not match patients appearance ① To measure ulna length – Complete Women Men this once, on admission Ulna Under 65 years Ulna Under 65 Ensure the patients left arm is bare length 65 & over length 65 years years from palm to elbow (cm) years (cm) & over Ask the patient Approximate Approximate to cross their height (metres) height (metres) left arm across 32.0 1.84 1.84 32.0 1.94 1.87 their chest 31.5 1.83 1.83 31.5 1.93 1.86 (as in this 31.0 1.81 1.81 31.0 1.91 1.84 picture) 30.5 1.80 1.79 30.5 1.89 1.82 30.0 1.79 1.78 30.0 1.87 1.81 Measure between the point of the 29.5 1.77 1.76 29.5 1.85 1.79 elbow (olecranon process) and the 29.0 1.76 1.75 29.0 1.84 1.78 midpoint of the prominent bone of 28.5 1.75 1.73 28.5 1.82 1.76 the wrist (styloid process) 28.0 1.73 1.71 28.0 1.80 1.75 Record ulna length on MUST chart 27.5 1.72 1.70 27.5 1.78 1.73 27.0 1.70 1.68 27.0 1.76 1.71 ② To find estimated height from ulna 26.5 1.69 1.66 26.5 1.75 1.70 length – Complete this once, on 26.0 1.68 1.65 26.0 1.73 1.68 admission 25.5 1.66 1.63 25.5 1.71 1.67 Follow a. -
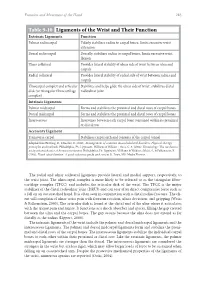
Table 9-10 Ligaments of the Wrist and Their Function
Function and Movement of the Hand 283 Table 9-10 Ligaments of the Wrist and Their Function Extrinsic Ligaments Function Palmar radiocarpal Volarly stabilizes radius to carpal bones; limits excessive wrist extension Dorsal radiocarpal Dorsally stabilizes radius to carpal bones; limits excessive wrist flexion Ulnar collateral Provides lateral stability of ulnar side of wrist between ulna and carpals Radial collateral Provides lateral stability of radial side of wrist between radius and carpals Ulnocarpal complex and articular Stabilizes and helps glide the ulnar side of wrist; stabilizes distal disk (or triangular fibrocartilage radioulnar joint complex) Intrinsic Ligaments Palmar midcarpal Forms and stabilizes the proximal and distal rows of carpal bones Dorsal midcarpal Forms and stabilizes the proximal and distal rows of carpal bones Interosseous Intervenes between each carpal bone contained within its proximal or distal row Accessory Ligament Transverse carpal Stabilizes carpal arch and contents of the carpal tunnel Adapted from Hertling, D., & Kessler, R. (2006). Management of common musculoskeletal disorders: Physical therapy principles and methods. Philadelphia, PA: Lippincott, Williams & Wilkins.; Oatis, C. A. (2004). Kinesiology: The mechanics and pathomechanics of human movement. Philadelphia, PA: Lippincott, Williams & Wilkins.; Weiss, S., & Falkenstein, N. (2005). Hand rehabilitation: A quick reference guide and review. St. Louis, MO: Mosby Elsevier. The radial and ulnar collateral ligaments provide lateral and medial support, respectively, to the wrist joint. The ulnocarpal complex is more likely to be referred to as the triangular fibro- cartilage complex (TFCC) and includes the articular disk of the wrist. The TFCC is the major stabilizer of the distal radioulnar joint (DRUJ) and can tear after direct compressive force such as a fall on an outstretched hand. -

The Appendicular Skeleton Appendicular Skeleton
THE SKELETAL SYSTEM: THE APPENDICULAR SKELETON APPENDICULAR SKELETON The primary function is movement It includes bones of the upper and lower limbs Girdles attach the limbs to the axial skeleton SKELETON OF THE UPPER LIMB Each upper limb has 32 bones Two separate regions 1. The pectoral (shoulder) girdle (2 bones) 2. The free part (30 bones) THE PECTORAL (OR SHOULDER) GIRDLE UPPER LIMB The pectoral girdle consists of two bones, the scapula and the clavicle The free part has 30 bones 1 humerus (arm) 1 ulna (forearm) 1 radius (forearm) 8 carpals (wrist) 19 metacarpal and phalanges (hand) PECTORAL GIRDLE - CLAVICLE The clavicle is “S” shaped The medial end articulates with the manubrium of the sternum forming the sternoclavicular joint The lateral end articulates with the acromion forming the acromioclavicular joint THE CLAVICLE PECTORAL GIRDLE - CLAVICLE The clavicle is convex in shape anteriorly near the sternal junction The clavicle is concave anteriorly on its lateral edge near the acromion CLINICAL CONNECTION - FRACTURED CLAVICLE A fall on an outstretched arm (F.O.O.S.H.) injury can lead to a fractured clavicle The clavicle is weakest at the junction of the two curves Forces are generated through the upper limb to the trunk during a fall Therefore, most breaks occur approximately in the middle of the clavicle PECTORAL GIRDLE - SCAPULA Also called the shoulder blade Triangular in shape Most notable features include the spine, acromion, coracoid process and the glenoid cavity FEATURES ON THE SCAPULA Spine - -

Bone Limb Upper
Shoulder Pectoral girdle (shoulder girdle) Scapula Acromioclavicular joint proximal end of Humerus Clavicle Sternoclavicular joint Bone: Upper limb - 1 Scapula Coracoid proc. 3 angles Superior Inferior Lateral 3 borders Lateral angle Medial Lateral Superior 2 surfaces 3 processes Posterior view: Acromion Right Scapula Spine Coracoid Bone: Upper limb - 2 Scapula 2 surfaces: Costal (Anterior), Posterior Posterior view: Costal (Anterior) view: Right Scapula Right Scapula Bone: Upper limb - 3 Scapula Glenoid cavity: Glenohumeral joint Lateral view: Infraglenoid tubercle Right Scapula Supraglenoid tubercle posterior anterior Bone: Upper limb - 4 Scapula Supraglenoid tubercle: long head of biceps Anterior view: brachii Right Scapula Bone: Upper limb - 5 Scapula Infraglenoid tubercle: long head of triceps brachii Anterior view: Right Scapula (with biceps brachii removed) Bone: Upper limb - 6 Posterior surface of Scapula, Right Acromion; Spine; Spinoglenoid notch Suprspinatous fossa, Infraspinatous fossa Bone: Upper limb - 7 Costal (Anterior) surface of Scapula, Right Subscapular fossa: Shallow concave surface for subscapularis Bone: Upper limb - 8 Superior border Coracoid process Suprascapular notch Suprascapular nerve Posterior view: Right Scapula Bone: Upper limb - 9 Acromial Clavicle end Sternal end S-shaped Acromial end: smaller, oval facet Sternal end: larger,quadrangular facet, with manubrium, 1st rib Conoid tubercle Trapezoid line Right Clavicle Bone: Upper limb - 10 Clavicle Conoid tubercle: inferior -
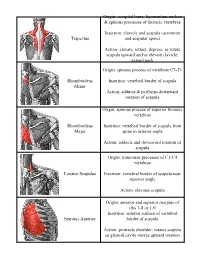
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

2.0 Mm LCP Distal Ulna Plate TG
2.0 mm LCP Distal Ulna Plate. For capital and subcapital fractures of the ulna. Technique Guide Table of Contents Introduction 2.0 mm LCP Distal Ulna Plate 2 Indications 4 Surgical Technique Clinical Examples 5 Approach 6 Reduce Fracture and Position Plate 7 Fix Plate Distally 9 Adjust Length and Complete Fixation 11 Closure 13 Implant Removal 13 Product Information Implants 14 Instruments 16 IMPORTANT: This device has not been evaluated for safety and compatibility in the MR environment. This device has not been tested for heating or migration in the MR environment. Image intensifier control Synthes 2.0 mm LCP Distal Ulna Plate. For capital and subcapital fractures of the ulna. The distal ulna is an essential component of the distal radioulnar joint, which helps provide rotation to the forearm. The distal ulnar surface is also an important platform for stability of the carpus and, beyond it, the hand. Unstable fractures of the distal ulna therefore threaten both movement and stability of the wrist. The size and shape of the distal ulna, combined with the overlying mobile soft tissues, make application of standard implants difficult. The 2.0 mm LCP Distal Ulna Plate is specifically designed for use in fractures of the distal ulna. Features – Pointed hooks and locking screws in the head – Anatomically precontoured – Angular stability 2 Synthes 2.0 mm LCP Distal Ulna Plate Technique Guide Narrow plate design, low screw-plate profile, rounded edges and polished surface are designed to minimize irritation of overlying soft tissue Pointed hooks -
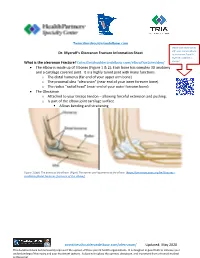
Olecranon Fractures Diagnosed? • the First Thing I Do Is Listen to Your Story
Twincitiesshoulderandelbow.com Please scan these codes with your camera phone Dr. Myeroff’s Olecranon Fracture Information Sheet to learn more from Dr. Myeroff’s website as What is the olecranon Fracture? twincitiesshoulderandelbow.com/elbowfracturevideo/ you go! • The elbow is made up of 3 bones (Figure 1 & 2): Each bone has complex 3D anatomy and a cartilage covered joint. It is a highly tuned joint with many functions. o The distal humerus (far end of your upper arm bone) o The proximal ulna “olecranon” (near end of your inner forearm bone) o The radius “radial head” (near end of your outer forearm bone) • The Olecranon o Attached to your triceps tendon – allowing forceful extension and pushing. o Is part of the elbow joint cartilage surface ▪ Allows bending and straitening Figure 1(Left) The bones of the elbow. (Right) The nerves and ligaments of the elbow. (https://orthoinfo.aaos.org/en/diseases-- conditions/distal-humerus-fractures-of-the-elbow/) twincitiesshoulderandelbow.com/olecranon/ Updated: May 2020 This document does not necessarily represent the opinion of these parent health organizations. It is designed in good faith to increase your understanding of this injury and your treatment options. It does not replace the opinion, discussion, and treatment from a trained medical professional. Figure 2Elbow viewed directly from the front (left) and back (right). Figure 3(Left) Medial elbow ligaments (Right) Lateral elbow ligaments • Elbow injuries are at risk of two conflicting outcomes o Stiffness – because of its complex anatomy, the elbow is famous for stiffness after injuries. ▪ After the first 3 months from injury, it is incredibly hard or impossible to obtain more motion in the elbow without a surgery. -
Both-Bone Forearm Fracture with Distal Radioulnar Joint Dislocation
A Case Report & Literature Review Both-Bone Forearm Fracture With Distal Radioulnar Joint Dislocation Michael K. Ryan, MD, Brendan J. MacKay, MD, and Nirmal C. Tejwani, MD and a concomitant ipsilateral DRUJ dislocation, and we review Abstract the literature on this uncommon injury pattern. Both-bone forearm fractures, also known as The patient provided written informed consent for print concomitant diaphyseal radius and ulna frac- and electronic publication of this case report. tures, and distal radioulnar joint (DRUJ) disloca- tions are each uncommon injuries in adult upper Case Report extremity trauma. DRUJ dislocations are more A 38-year-old man with no past medical history presented to often associated with radial shaft (Galeazzi) the emergency department after being thrown from a motor- fractures. In this article, we report the case of cycle. On arrival, he reported bilateral upper extremity pain. a patient who sustained a both-bone forearm Physical examination revealed closed deformities of the left fracture and DRUJ dislocation, and we review forearm and right wrist. Radiographs of the left upper extrem- the literature on this unique injury pattern. ity showed mid-diaphyseal radius and ulna fractures and a DRUJ dislocation (Figure 1). Radiographs of the right upper extremity showed a comminuted radial styloid fracture and widening of the scapholunate interval with dorsal interca- iaphyseal forearm fractures are relatively rare in or- lated segment instability deformity of the carpus. Both upper thopedic trauma, accounting for only 0.9% of all frac- extremity injuries were acceptably reduced and splinted. The 1 AJO Dtures. Both-bone forearm fractures, also known as patient also had a left-side subarachnoid hematoma, which concomitant diaphyseal radius and ulna fractures, are even was stable on serial computed tomography without contrast. -
Muscles of the Upper Limb.Pdf
11/8/2012 Muscles Stabilizing Pectoral Girdle Muscles of the Upper Limb Pectoralis minor ORIGIN: INNERVATION: anterior surface of pectoral nerves ribs 3 – 5 ACTION: INSERTION: protracts / depresses scapula coracoid process (scapula) (Anterior view) Muscles Stabilizing Pectoral Girdle Muscles Stabilizing Pectoral Girdle Serratus anterior Subclavius ORIGIN: INNERVATION: ORIGIN: INNERVATION: ribs 1 - 8 long thoracic nerve rib 1 ---------------- INSERTION: ACTION: INSERTION: ACTION: medial border of scapula rotates scapula laterally inferior surface of scapula stabilizes / depresses pectoral girdle (Lateral view) (anterior view) Muscles Stabilizing Pectoral Girdle Muscles Stabilizing Pectoral Girdle Trapezius Levator scapulae ORIGIN: INNERVATION: ORIGIN: INNERVATION: occipital bone / spinous accessory nerve transverse processes of C1 – C4 dorsal scapular nerve processes of C7 – T12 ACTION: INSERTION: ACTION: INSERTION: stabilizes / elevates / retracts / upper medial border of scapula elevates / adducts scapula acromion / spine of scapula; rotates scapula lateral third of clavicle (Posterior view) (Posterior view) 1 11/8/2012 Muscles Stabilizing Pectoral Girdle Muscles Moving Arm Rhomboids Pectoralis major (major / minor) ORIGIN: INNERVATION: ORIGIN: INNERVATION: spinous processes of C7 – T5 dorsal scapular nerve sternum / clavicle / ribs 1 – 6 dorsal scapular nerve INSERTION: ACTION: INSERTION: ACTION: medial border of scapula adducts / rotates scapula intertubucular sulcus / greater tubercle flexes / medially rotates / (humerus) adducts -

Right Or Left Bones? Clavicle Ulna
Right or Left Bones? Clavicle Ulna . Together, the clavicles make a . Lay the ulna on the table. “handlebar” shape (low at the middle . Can you see the radial notch? of the chest, higher near the shoulders). o If yes, the open side of the . The blunt tip (sternal end) touches the trochlear (semilunar) notch tells sternum at the middle of the chest. you the bone orientation (see . The round tip (acromial end) touches the photo 1). scapula at the shoulder. o If no, the open side of the . The bumpy side of the clavicle faces the trochlear (semilunar) notch is the rib cage. opposite of the bone orientation . The smooth side of the clavicle faces (see photo 2). outward Radius Fibula . The styloid process always touches the . The smoother tip (head of the fibula) thumb. touches the knee. If the lower end of the radius (near the . The rougher tip (lateral malleolus) styloid process) is rough, you are touches the ankle. looking at the back of the wrist. The point of the lateral malleolus points . If the lower end of the radius is smooth, to the pinkie toe, not the middle of the you are looking at the inside of the wrist. foot. The front of the fibula (anterior view) has an edge. The back of the fibula (posterior view) is flat. References: University of Liverpool Faculty of Health and Life Sciences. (2013). Radius and ulna (right forearm) [Digital photograph]. Retrieved from https://www.flickr.com/photos/liverpoolhls/10819145494. . -

Surgical Management of Ulnar Styloid Fractures
Chen et al. Journal of Orthopaedic Surgery and Research (2020) 15:273 https://doi.org/10.1186/s13018-020-01795-3 RESEARCH ARTICLE Open Access Surgical management of ulnar styloid fractures: comparison of fixation with anchor suture and tension band wire Alvin Chao-Yu Chen* , Yi-Hsuan Lin, Chun-Jui Weng and Chun-Ying Cheng Abstract Background: Limited reference is available regarding surgical management in symptomatic ulnar styloid fractures with small bony avulsion. The study goal is to report the surgical outcomes using anchor suture fixation with comparison to traditional tension band wire fixation. Methods: We retrospectively reviewed the medical records in patients who underwent surgical repair for unilateral ulnar styloid fractures with distal radioulnar instability between 2004 and 2017. A total of 31 patients were enrolled including two kinds of fixation methods. Anchor suture fixation plus distal radioulnar joint pinning was performed in ten patients with tiny avulsion bony fragments (group A); tension band wire fixation was performed in 21 patients with big styloid fracture fragments (group B). Patient characteristics and 2-year treatment outcomes were compared between two groups based on Mayo Modified Wrist Score (MMWS); Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH); visual analog scale (VAS), and surgical complication. Descriptive statistics were used for calculation of key variables; a p value of < 0.05 was considered statistically significant. Results: Based on Gaulke classification, there were five subtypes in group A and three subtypes in group B. Incidence of concomitant distal radius fractures was significantly higher in group B; other patient characteristics including age, sex, injury side, and time to surgery showed no significant difference. -

Age and Sex Estimation from the Human Clavicle: an Investigation of Traditional and Novel Methods
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: AGE AND SEX ESTIMATION FROM THE HUMAN CLAVICLE: AN INVESTIGATION OF TRADITIONAL AND NOVEL METHODS Author: Natalie Renee Shirley Document No.: 227930 Date Received: August 2009 Award Number: 2007-DN-BX-0004 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant final report available electronically in addition to traditional paper copies. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. AGE AND SEX ESTIMATION FROM THE HUMAN CLAVICLE: AN INVESTIGATION OF TRADITIONAL AND NOVEL METHODS A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Natalie Renee Shirley May 2009 i This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. I dedicate this dissertation to Linda Finley, because without her none of this would have ever happened.