Genomic, Transcriptomic and Functional Studies of Diplomonads
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Morphology, Ultrastructure and Molecular Phylogeny of a New Freshwater Heterolobose Amoeba Parafumarolamoeba Stagnalis N. Sp
diversity Article The Morphology, Ultrastructure and Molecular Phylogeny of a New Freshwater Heterolobose Amoeba Parafumarolamoeba stagnalis n. sp. (Vahlkampfiidae; Heterolobosea) Anastasia S. Borodina 1,2, Alexander P. Mylnikov 1,†, Jan Janouškovec 3 , Patrick J. Keeling 4 and Denis V. Tikhonenkov 1,5,* 1 Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences, 152742 Borok, Russia; [email protected] 2 Department of Zoology and Parasitology, Voronezh State University, Universitetskaya Ploshad 1, 394036 Voronezh, Russia 3 Centre Algatech, Laboratory of Photosynthesis, Institute of Microbiology, Czech Academy of Sciences, Opatovický Mlýn, 37981 Tˇreboˇn,Czech Republic; [email protected] 4 Department of Botany, University of British Columbia, 6270 University Boulevard, Vancouver, BC V6T1Z4, Canada; [email protected] 5 AquaBioSafe Laboratory, University of Tyumen, 625003 Tyumen, Russia * Correspondence: [email protected]; Tel.: +7-485-472-4533 † Alexander P. Mylnikov is deceased. http://zoobank.org/References/e543a49a-16c1-4b7c-afdb-0bc56b632ef0 Abstract: Heterolobose amoebae are important members of marine, freshwater, and soil microbial Citation: Borodina, A.S.; Mylnikov, communities, but their diversity remains under-explored. We studied the diversity of Vahlkampfiidae A.P.; Janouškovec, J.; Keeling, P.J.; to improve our understanding of heterolobosean relationships and their representation in aquatic Tikhonenkov, D.V. The Morphology, benthos. Using light and electron microscopy, and molecular phylogenies based on the SSU rRNA Ultrastructure and Molecular and ITS loci, we describe the fine morphology and evolutionary relationships of a new heterolobosean Phylogeny of a New Freshwater Parafumarolamoeba stagnalis n. sp. from a small pond in European Russia. Cells of P. stagnalis possess Heterolobose Amoeba a clearly distinguishable anterior hyaline pseudopodium, eruptive movement, several thin and Parafumarolamoeba stagnalis n. -

Introgression and Hybridization in Animal Parasites
Genes 2010, 1, 102-123; doi:10.3390/genes1010102 OPEN ACCESS genes ISSN 2073-4425 www.mdpi.com/journal/genes Review An Infectious Topic in Reticulate Evolution: Introgression and Hybridization in Animal Parasites Jillian T. Detwiler * and Charles D. Criscione Department of Biology, Texas A&M University, 3258 TAMU, College Station, TX 77843, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-979-845-0925; Fax: +1-979-845-2891. Received: 29 April 2010; in revised form: 7 June 2010 / Accepted: 7 June 2010 / Published: 9 June 2010 Abstract: Little attention has been given to the role that introgression and hybridization have played in the evolution of parasites. Most studies are host-centric and ask if the hybrid of a free-living species is more or less susceptible to parasite infection. Here we focus on what is known about how introgression and hybridization have influenced the evolution of protozoan and helminth parasites of animals. There are reports of genome or gene introgression from distantly related taxa into apicomplexans and filarial nematodes. Most common are genetic based reports of potential hybridization among congeneric taxa, but in several cases, more work is needed to definitively conclude current hybridization. In the medically important Trypanosoma it is clear that some clonal lineages are the product of past hybridization events. Similarly, strong evidence exists for current hybridization in human helminths such as Schistosoma and Ascaris. There remain topics that warrant further examination such as the potential hybrid origin of polyploid platyhelminths. -

Pronunciation Guide to Microorganisms
Pronunciation Guide to Microorganisms This pronunciation guide is provided to aid each student in acquiring a greater ease in discussing, describing, and using specific microorganisms. Please note that genus and species names are italicized. If they cannot be italicized, then they should be underlined (example: a lab notebook). Prokaryotic Species Correct Pronunciation Acetobacter aceti a-se-toh-BAK-ter a-SET-i Acetobacter pasteurianus a-se-toh-BAK-ter PAS-ter-iann-us Acintobacter calcoacetius a-sin-ee-toe-BAK-ter kal-koh-a-SEE-tee-kus Aerococcus viridans (air-o)-KOK-kus vi-ree-DANS Agrobacterium tumefaciens ag-roh-bak-TEAR-ium too-me-FAY-she-ens Alcaligenes denitrificans al-KAHL-li-jen-eez dee-ni-TREE-fee-cans Alcaligenes faecalis al-KAHL-li-jen-eez fee-KAL-is Anabaena an-na-BEE-na Azotobacter vinelandii a-zoe-toe-BAK-ter vin-lan-DEE-i Bacillus anthracis bah-SIL-lus AN-thray-sis Bacillus lactosporus bah-SIL-lus LAK-toe-spore-us Bacillus megaterium bah-SIL-lus Meg-a-TEER-ee-um Bacillus subtilis bah-SIL-lus SA-til-us Borrelia recurrentis bore-RELL-ee-a re-kur-EN-tis Branhamella catarrhalis bran-hem-EL-ah cat-arr-RAH-lis Citrobacter freundii sit-roe-BACK-ter FROND-ee-i Clostridium perfringens klos-TREH-dee-um per-FRINGE-enz Clostridium sporogenes klos-TREH-dee-um spore-AH-gen-ease Clostridium tetani klos-TREH-dee-um TET-ann-ee Corynebacterium diphtheriae koh-RYNE-nee-back-teer-ee-um dif-THEE-ry-ee Corynebacterium hofmanni koh-RYNE-nee-back-teer-ee-um hoff-MAN-eye Corynebacterium xerosis koh-RYNE-nee-back-teer-ee-um zer-OH-sis Enterobacter -

3.2.2 Diplomonad (Hexamitid) Flagellates - 1
3.2.2 Diplomonad (Hexamitid) Flagellates - 1 3.2.2 Diplomonad (Hexamitid) Flagellates: Diplomonadiasis, Hexamitosis, Spironucleosis Sarah L. Poynton Department of Comparative Medicine Johns Hopkins University School of Medicine 1-127 Jefferson Building, Johns Hopkins Hospital 600 North Wolfe Street Baltimore, MD 21287 410/502-5065 fax: 443/287-2954 [email protected] [email protected] A. Name of Disease and Etiological Agent Diplomonadiasis or hexamitosis is infection by diplomonad flagellates (Order Diplomonadida, suborder Diplomonadina, Family Hexamitidae). If the exact genus is known, the infections may be reported as hexamitiasis (Hexamita), octomitosis (Octomitus), or spironucleosis (Spironucleus); of these, probably only the latter is applicable to fish (see below). Infections may be reported as localized (commonly in the intestine, and possibly also including “hole-in-the head disease” of cichlids (Paull and Matthews 2001), or disseminated or systemic (Ferguson and Moccia 1980; Kent et al. 1992; Poppe et al. 1992; Sterud et al. 1998). Light microscopy studies have reported three genera from fish - namely Hexamita, Octomitus, and Spironucleus. However, transmission electron microscopy (TEM) is needed to confirm genus (Poynton and Sterud 2002), and light microscopy studies are therefore taxonomically unreliable. If TEM is not available, the organisms should be recorded as diplomonad or hexamitid flagellates. All recent comprehensive ultrastructural studies show only the genus Spironucleus infecting fish, and it is probable that this is the genus to which all diplomonads from fish belong (Poynton and Sterud 2002). Some 15 to 20 species of diplomonads have been reported from fish (Poynton and Sterud 2002). However, most descriptions do not include comprehensive surface and internal ultrastructure and thus are incomplete. -

Cyprinus Carpio
Cyprinus carpio Investigation of infection by some Endo- parasitic Protozoa species in common carp ( Cyprinus carpio ) in AL-Sinn fishfarm 2014 Cyprinus carpio Investigation of infection by some Endo- parasitic Protozoa species in common carp ( Cyprinus carpio ) in AL-Sinn fishfarm 2014 I IV V VI VII 2 1 3 2 3 3 4 4 4 1 4 4 1 1 4 2 4 6 6 1 2 4 6 6 7 8 8 10 12 14 15 15 16 2 2 4 I 16 16 16 17 17 18 18 19 19 19 Hexamitosis 3 2 4 19 19 20 20 21 21 21 1 3 4 22 23 2 3 4 26 5 26 1 5 27 2 5 28 3 5 29 4 5 29 1 4 5 29 2 4 5 33 3 4 5 II 34 4 4 5 34 5 4 5 36 6 36 1 6 42 2 6 42 Hexamitosis 3 6 43 7 44 8 44 9 49 10 49 1 10 51 2 10 III 5 1 Oocyst 7 2 15 3 11 Merozoites 4 12 5 12 6 13 7 14 8 15 9 Trypanosoma 1 18 10 Trypanoplasma 2 19 11 21 Hexamita 12 23 Hexamita 13 A 28 14 B 30 Cyprinus carpio carpio 15 31 16 33 17 34 18 34 19 35 20 35 21 36 22 IV 40 Goussia carpelli 23 40 Macrogametocyte 24 41 Merozoites 25 37 1 Goussia carpelli 39 2 Goussia 41 3 carpelli Goussia 42 carpelli 4 43 5 Goussia 44 carpelli 6 V Goussia Trypanosoma Trypanoplasma borreli Goussia subepithelialis carpelli Hexamita intestinalis danilewskyi Hexamitosis 200 2013 / 5 / 14 2012 / 6 / 6 Goussia carpelli Goussia subepithelials Nodular Coccidiosis Macrogametocytes Merozoites 3 Goussia carpelli 5.4 1.6 17.6 95.84 17.5 16 Trypanosoma Trypanoplasma borreli Hexamita intestinalis danilewskyi VI Abstract This study aimed at investigating the infection of cultured common carp ( Cyprinus carpio L. -

Multigene Eukaryote Phylogeny Reveals the Likely Protozoan Ancestors of Opis- Thokonts (Animals, Fungi, Choanozoans) and Amoebozoa
Accepted Manuscript Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opis- thokonts (animals, fungi, choanozoans) and Amoebozoa Thomas Cavalier-Smith, Ema E. Chao, Elizabeth A. Snell, Cédric Berney, Anna Maria Fiore-Donno, Rhodri Lewis PII: S1055-7903(14)00279-6 DOI: http://dx.doi.org/10.1016/j.ympev.2014.08.012 Reference: YMPEV 4996 To appear in: Molecular Phylogenetics and Evolution Received Date: 24 January 2014 Revised Date: 2 August 2014 Accepted Date: 11 August 2014 Please cite this article as: Cavalier-Smith, T., Chao, E.E., Snell, E.A., Berney, C., Fiore-Donno, A.M., Lewis, R., Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts (animals, fungi, choanozoans) and Amoebozoa, Molecular Phylogenetics and Evolution (2014), doi: http://dx.doi.org/10.1016/ j.ympev.2014.08.012 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 1 Multigene eukaryote phylogeny reveals the likely protozoan ancestors of opisthokonts 2 (animals, fungi, choanozoans) and Amoebozoa 3 4 Thomas Cavalier-Smith1, Ema E. Chao1, Elizabeth A. Snell1, Cédric Berney1,2, Anna Maria 5 Fiore-Donno1,3, and Rhodri Lewis1 6 7 1Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK. -

The Intestinal Protozoa
The Intestinal Protozoa A. Introduction 1. The Phylum Protozoa is classified into four major subdivisions according to the methods of locomotion and reproduction. a. The amoebae (Superclass Sarcodina, Class Rhizopodea move by means of pseudopodia and reproduce exclusively by asexual binary division. b. The flagellates (Superclass Mastigophora, Class Zoomasitgophorea) typically move by long, whiplike flagella and reproduce by binary fission. c. The ciliates (Subphylum Ciliophora, Class Ciliata) are propelled by rows of cilia that beat with a synchronized wavelike motion. d. The sporozoans (Subphylum Sporozoa) lack specialized organelles of motility but have a unique type of life cycle, alternating between sexual and asexual reproductive cycles (alternation of generations). e. Number of species - there are about 45,000 protozoan species; around 8000 are parasitic, and around 25 species are important to humans. 2. Diagnosis - must learn to differentiate between the harmless and the medically important. This is most often based upon the morphology of respective organisms. 3. Transmission - mostly person-to-person, via fecal-oral route; fecally contaminated food or water important (organisms remain viable for around 30 days in cool moist environment with few bacteria; other means of transmission include sexual, insects, animals (zoonoses). B. Structures 1. trophozoite - the motile vegetative stage; multiplies via binary fission; colonizes host. 2. cyst - the inactive, non-motile, infective stage; survives the environment due to the presence of a cyst wall. 3. nuclear structure - important in the identification of organisms and species differentiation. 4. diagnostic features a. size - helpful in identifying organisms; must have calibrated objectives on the microscope in order to measure accurately. -

Primary Amoebic Meningoencephalitis Due to Naegleria Fowleri
56 Case report Primary amoebic meningoencephalitis due to Naegleria fowleri A. Angrup, L. Chandel, A. Sood, K. Thakur, S. C. Jaryal Department of Microbiology,Dr. Rajendra Prasad Government Medical College, Kangra at Tanda, Himachal Pradesh, Pin Code- 176001, India. Correspondence to: Dr. Archana Angrup, Department of Microbiology, Dr. Rajendra Prasad Government Medical College, Kangra, Tanda, Himachal Pradesh, Pin Code-176001, India. Phone no. 09418119222, Facsimile: 01892-267115 Email: [email protected] Abstract The genus Naegleria comprises of free living ameboflagellates found in soil and fresh water. More than 30 species have been isolated but only N. fowleri has been associated with human disease. N. fowleri causes primary amoebic meningoencephalitis (PAM), an acute, often fulminant infection of CNS. Here we report a rare and first case of PAM in an immunocompetent elderly patient from this part of the country. Amoeboid and flagellate forms of N. fowleri were detected in the direct microscopic examination of CSF and confirmed by flagellation test in distilled water, demonstrating plaques /clear areas on 1.5% non nutrient agar and its survival at 42°C. Keywords: Meningitis, Naegleria fowleri, primary amoebic meningoencephalitis Introduction of our knowledge, in India, only eight cases have been reported so far .1, 5-8 Infection of the central nervous system (CNS) in human We hereby report a rare case of PAM in elderly beings with free living amoebae is uncommon. Among the immunocompetent patient from the hilly state of Himachal many different genera of amoebae, Naegleria spp, Pradesh (H.P) in Northern India. Acanthamoeba spp and Balamuthia spp are primarily pathogenic to the CNS. -

A Wide Diversity of Previously Undetected Freeliving
Environmental Microbiology (2010) 12(10), 2700–2710 doi:10.1111/j.1462-2920.2010.02239.x A wide diversity of previously undetected free-living relatives of diplomonads isolated from marine/saline habitatsemi_2239 2700..2710 Martin Kolisko,1 Jeffrey D. Silberman,2 Kipferlia n. gen. The remaining isolates include rep- Ivan Cepicka,3 Naoji Yubuki,4† Kiyotaka Takishita,5 resentatives of three other lineages that likely repre- Akinori Yabuki,4 Brian S. Leander,6 Isao Inouye,4 sent additional undescribed genera (at least). Small- Yuji Inagaki,7 Andrew J. Roger8 and subunit ribosomal RNA gene phylogenies show that Alastair G. B. Simpson1* CLOs form a cloud of six major clades basal to the Departments of 1Biology and 8Biochemistry and diplomonad-retortamonad grouping (i.e. each of the Molecular Biology, Dalhousie University, Halifax, Nova six CLO clades is potentially as phylogenetically Scotia, Canada. distinct as diplomonads and retortamonads). CLOs 2Department of Biological Sciences, University of will be valuable for tracing the evolution of Arkansas, Fayetteville, AR, USA. diplomonad cellular features, for example, their 3Department of Zoology, Faculty of Science, Charles extremely reduced mitochondrial organelles. It is University in Prague, Prague, Czech Republic. striking that the majority of CLO diversity was unde- 4Institute of Biological Sciences, Graduate School of Life tected by previous light microscopy surveys and and Environmental Sciences and 7Center for environmental PCR studies, even though they inhabit Computational Sciences and Institute of Biological a commonly sampled environment. There is no Sciences, University of Tsukuba, Tsukuba, Ibaraki, reason to assume this is a unique situation – it is Japan. likely that undersampling at the level of major lin- 5Japan Agency for Marine-Earth Science and eages is still widespread for protists. -
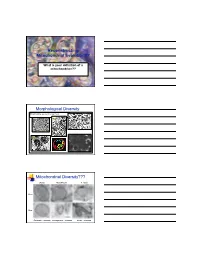
Reconstructing Mitochondrial Evolution?? Morphological Diversity Mitochondrial Diversity???
Reconstructing Mitochondrial Evolution?? What is your definition of a mitochondrion?? Morphological Diversity Mitochondria as we all know them: Suprarenal gland Liver cell Plasma cell Adrenal cortex Mitochondrial Diversity??? Chicken Neocallimastix T. foetus 100 nm 50 nm Entamoeba - mitosome microsporidian - mitosome Giardia - mitosome Reconstructing Evolution Mitochondrial evolution well established endosymbiotic theory α-proteobacterium - Rickettsia prowazekii Hydrogenosomal evolution No DNA NOW 2 examples Nyctotherus and Blastocystis (MLO) Several proteins similar to mitochondria Mitosome evolution No DNA Few proteins identified similar to mitochondria Origins via Endosymbiosis Current dogma - mitochondria and related Aerobic α-proteobacterium prokaryote gave rise to present day mitochondria. organelles arose just once in evolution Are hydrogenosomes and mitosomes of anaerobic protists derived from the same proto-mitochondrion? Evidence for: accumulating evidence for several proteins that are currently found in mitochondria - Proteins of Fe-S cluster formation. Scenario A Common ancestor facultative Scenario B organism? degenerate mitochondrion invoke lateral gene transfer from anaerobic prokaryotes Hypotheses for Mito Acquisition Mitochondrion-related Organelles 2 way to interpret this summary - can you think of both? Groups with mitochondrial homologues Hjort et al Phil. Trans. R. Soc. B 2010 365, 713-727 Origin of Hydrogenosomes (Mitosomes?) 1. a)Conversion of Mitochondria HYD b)Common ancestor with mitochondria ? HYD 2. Independent Origin α-proteobacterium ? - anaerobic bacterium HYD Organelles - origins and biogenesis Approaches: (1) Conduct phylogenetic analyses of similar proteins Hsp70 Fd Hsp60 Isc subunits (2) Examine protein targeting to the organelle matrix protein targeting membrane protein targeting (3) Characterize membrane/translocation components These components could have evolved as the endosymbiont was converted to organelle. Reveals evolutionary history. -

Protist Phylogeny and the High-Level Classification of Protozoa
Europ. J. Protistol. 39, 338–348 (2003) © Urban & Fischer Verlag http://www.urbanfischer.de/journals/ejp Protist phylogeny and the high-level classification of Protozoa Thomas Cavalier-Smith Department of Zoology, University of Oxford, South Parks Road, Oxford, OX1 3PS, UK; E-mail: [email protected] Received 1 September 2003; 29 September 2003. Accepted: 29 September 2003 Protist large-scale phylogeny is briefly reviewed and a revised higher classification of the kingdom Pro- tozoa into 11 phyla presented. Complementary gene fusions reveal a fundamental bifurcation among eu- karyotes between two major clades: the ancestrally uniciliate (often unicentriolar) unikonts and the an- cestrally biciliate bikonts, which undergo ciliary transformation by converting a younger anterior cilium into a dissimilar older posterior cilium. Unikonts comprise the ancestrally unikont protozoan phylum Amoebozoa and the opisthokonts (kingdom Animalia, phylum Choanozoa, their sisters or ancestors; and kingdom Fungi). They share a derived triple-gene fusion, absent from bikonts. Bikonts contrastingly share a derived gene fusion between dihydrofolate reductase and thymidylate synthase and include plants and all other protists, comprising the protozoan infrakingdoms Rhizaria [phyla Cercozoa and Re- taria (Radiozoa, Foraminifera)] and Excavata (phyla Loukozoa, Metamonada, Euglenozoa, Percolozoa), plus the kingdom Plantae [Viridaeplantae, Rhodophyta (sisters); Glaucophyta], the chromalveolate clade, and the protozoan phylum Apusozoa (Thecomonadea, Diphylleida). Chromalveolates comprise kingdom Chromista (Cryptista, Heterokonta, Haptophyta) and the protozoan infrakingdom Alveolata [phyla Cilio- phora and Miozoa (= Protalveolata, Dinozoa, Apicomplexa)], which diverged from a common ancestor that enslaved a red alga and evolved novel plastid protein-targeting machinery via the host rough ER and the enslaved algal plasma membrane (periplastid membrane). -

Identification of the Meiotic Life Cycle Stage of Trypanosoma Brucei in The
Identification of the meiotic life cycle stage of Trypanosoma brucei in the tsetse fly Lori Peacocka,b, Vanessa Ferrisa,b, Reuben Sharmac,1, Jack Sunterc, Mick Baileyb, Mark Carringtonc, and Wendy Gibsona,2 aSchool of Biological Sciences, University of Bristol, Bristol BS8 1UG, United Kingdom; bDepartment of Clinical Veterinary Science, University of Bristol, Bristol BS40 7DU, United Kingdom; and cDepartment of Biochemistry, University of Cambridge, Cambridge CB2 1QW, United Kingdom Edited by Francisco J. Ayala, University of California, Irvine, CA, and approved January 27, 2011 (received for review December 23, 2010) Elucidating the mechanism of genetic exchange is fundamental for genetic exchange in T. brucei involves mixing of mitochondrial understanding how genes for such traits as virulence, disease (kinetoplast) and nuclear genomes, because hybrid progeny have phenotype, and drug resistance are transferred between pathogen hybrid kinetoplast DNA (kDNA) networks with mini-circles de- strains. Genetic exchange occurs in the parasitic protists Trypano- rived from both parents (14, 15). Plausible models for the gen- soma brucei, T. cruzi, and Leishmania major, but the precise cellular eration of hybrid kDNA networks are limited by the complex mechanisms are unknown, because the process has not been ob- structure and highly ordered replication of this concatenated served directly. Here we exploit the identification of homologs of mass of small DNA circles (16). Finally, trypanosomes belong to meiotic genes in the T. brucei genome and demonstrate that three the Euglenozoa, a deep branch within the excavate eukaryote su- functionally distinct, meiosis-specific proteins are expressed in the pergroup (17, 18). The production of four haploid gametes and nucleus of a single specific cell type, defining a previously unde- subsequent fusion to reform the diploid occurring in trypanosomes scribed developmental stage occurring within the tsetse flysalivary would strongly suggest the presence of a typical meiosis in the last gland.