Rockfish Conservation Areas in B.C.: Our Current State of Knowledge
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Come Celebrate! [email protected] 1-866-944-1744
Gulf Islands National Park Reserve parkscanada.gc.ca Come Celebrate! [email protected] 1-866-944-1744 Parks Pares Canada Canada Canada TABLE OF CONTENTS Contact Information 2 Welcome to Gulf Islands National Park Reserve, one of Programs 5 Top 10 Experiences 6-7 Canada's newest national parks. Established in 2003, it First Nations 8-9 Camping & Mooring 10 Trails 11 safeguards a portion of British Columbia's beautiful southern BC Ferries Coastal Naturalist Program 12 Gulf Islands in the Strait of Georgia. A mosaic of open Map 12-13 Species at Risk 14-15 meadows, forested hills, rocky headlands, quiet coves and Marine Wildlife Viewing 14-15 Extreme Take-Over 16 Did You Know? 17 sandy beaches, the park is a peaceful refuge just a stone's Ecological Integrity 17 Sidney Spit, D'Arcy throw from the urban clamour ofVancouver and Victoria. Island & Isle-de-Lis 18 Princess Margaret (Portland Is.), Brackman & Russell Islands 19 Pender Islands 20 Mayne Island 21 Saturna Island 22-23 Tumbo & Cabbage Islands 23 CONTACT INFORMATION Website information www.parkscanada.gc.ca/gulf Emergency and Important Phone Numbers Emergency call 911 In-Park Emergency or to report an offence 1-877-852-3100 Report a Wildfire 1-800-663-5555 (*5555 on cell phones) Marine Distress VHF Channel 16 Park Office • 250-654-4000 Toll Free 1-866-944-1744 Sidney Operations Centre 2220 Harbour Road Sidney, B.C. V8L 2P6 RCMP detachment offices located in Sidney, on the Penders, and on Mayne Island. Wflp\,t to teiA/OW pvu>re? The park offers many activities and learning opportunities. -

California Saltwater Sport Fishing Regulations
2017–2018 CALIFORNIA SALTWATER SPORT FISHING REGULATIONS For Ocean Sport Fishing in California Effective March 1, 2017 through February 28, 2018 13 2017–2018 CALIFORNIA SALTWATER SPORT FISHING REGULATIONS Groundfish Regulation Tables Contents What’s New for 2017? ............................................................. 4 24 License Information ................................................................ 5 Sport Fishing License Fees ..................................................... 8 Keeping Up With In-Season Groundfish Regulation Changes .... 11 Map of Groundfish Management Areas ...................................12 Summaries of Recreational Groundfish Regulations ..................13 General Provisions and Definitions ......................................... 20 General Ocean Fishing Regulations ��������������������������������������� 24 Fin Fish — General ................................................................ 24 General Ocean Fishing Fin Fish — Minimum Size Limits, Bag and Possession Limits, and Seasons ......................................................... 24 Fin Fish—Gear Restrictions ................................................... 33 Invertebrates ........................................................................ 34 34 Mollusks ............................................................................34 Crustaceans .......................................................................36 Non-commercial Use of Marine Plants .................................... 38 Marine Protected Areas and Other -

New Species, Corallivory, in Situ Video Observations and Overview of the Goniasteridae (Valvatida, Asteroidea) in the Hawaiian Region
Zootaxa 3926 (2): 211–228 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3926.2.3 http://zoobank.org/urn:lsid:zoobank.org:pub:39FE0179-9D06-4FC2-9465-CE69D79B933F New species, corallivory, in situ video observations and overview of the Goniasteridae (Valvatida, Asteroidea) in the Hawaiian Region CHRISTOPHER L. MAH Dept. of Invertebrate Zoology, Smithsonian Institution, Washington, D.C. 20007 Abstract Two new species of Goniasteridae, Astroceramus eldredgei n. sp. and Apollonaster kelleyi n. sp. are described from the Hawaiian Islands region. Prior to this occurrence, Apollonaster was known only from the North Atlantic. The Goniasteri- dae is the most diverse family of asteroids in the Hawaiian region. Additional in situ observations of several goniasterid species, including A. eldredgei n. sp. are reported. These observations extend documentation of deep-sea corallivory among goniasterid asteroids. New species occurrences presented herein suggested further biogeographic affinities be- tween tropical Pacific and Atlantic goniasterid faunas. Key words: Goniasteridae, Valvatida, deep-sea, Hawaiian Islands, predation Introduction Recent discoveries of new genera and species from deep-sea habitats along with new in situ video observations have provided us with new ecological insight into these poorly understood and formerly inaccessible settings (e.g., Mah et al. 2010, 2014; Mah & Foltz 2014). Hawaiian deep-sea Asteroidea are taxonomically diverse and occur in an active area of oceanographic and biological research (Chave and Malahoff 1998). New data on asteroids in this area presents an opportunity to review and highlight this diverse fauna. -
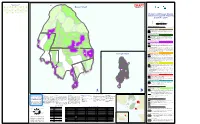
Bowyer and Passage Island Sensitive Ecosystem
480000m.E 482000m.E Sensitive and Terrestrial Ecosystems Label 368 391 460 1021* 1025 1026* 1027* 1028* 1030* CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 5YF:mx DC 5 5HB:ro RO 1 6MF:co HK 6 5HB:hb SC 1 8YF:mx HK 5 7MF:co HK 6 7MF:co HK 6 5HB:hb SC 1 8HB:ro RO 1 3NA RW 3HB:hb SC 1 3HB:ro RO 1 3HB:ro RO 1 1HB:ro RO 1 2YF:mx DC 5 2YF:mx DC 5 3HB:ro RO 1 2YF:mx DC 5 2HB:hb SC 1 2NA RW 1HB:hb SC 1 2NA RW 1NA RW 1NA RW 1NA RW 2YF:mx DC 5 Bowyer Island DSepRtembAer 30F, 201T0 1031 1032* 1035 1036 1037 1039 1040 1042 1043 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 6HB:ro RO 1 8MF:co HK 6 8YF:mx HK 5 8YF:mx HK 5 6YF:mx HK 5 5YF:co DC 5 6YF:co DC 5 8MF:co HK 6 7HB:hb SC 1 3NA BE 1 2YF:co DC 5 2MF:co HK 6 2MF:co HK 6 4MF:mx DF 6 3MF:co HK 6 2MF:co DF 6 2MF:co DF 6 3HB:ro RO 1 1HB:hb SC 1 2HB:hb SC 1 2HB:hb SC 1 1044 1045 1046 1048 1049 1050 1051 1052 1053 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 6MF:co HK 6 6MF:co DF 6 4YF:co DC 5 6MF:co HK 6 8MF:co HK 6 8MF:co HK 6 5YF:co DC 5 8MF:co HK 6 6MF:co HK 6 4MF:co DC 6 4MF:co HK 6 4YF:co HK 5 4MF:co RS 6 2MF:co HD 6 2MF:co RS 6 3YF:mx HK 5 2MF:co DC 6 4YF:mx HK 5 2HB:hb SC 1 2HB:hb SC 1 1054 1055* 1056 1057 1058 1059 1060 1061* CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 CWHxm1 10MF:co HK 6 7MF:co HK 6 6MF:co HK 6 10MF:co HK 6 7MF:co HK 6 7MF:co HK 6 10MF:co HK 6 8MF:co HK 6 3YF:co DC 5 4MF:co RS 6 3YF:co HK 5 3YF:co HK 5 2MF:co DF 6 1060 1061 Bowyer and Passage Islands 1059 Sensitive Ecosystem Mapping Airphoto - 2006 Scale: 1:15,000 UTM Projection Zone 10 NAD83 0 0.025 0.05 0.1 0.15 0.2 $ Kilometers 1057 1058 Sensitive Ecosystems Sensitive ecosystems are fragile and/or rare, or are ecologically important because of the diversity of species they support. -

Ucluelet Final
Culture and Heritage Study, Marine Resource Sites and Activities, Maa-nulth First Nations Ucluelet First Nation Project Final Report Halibut and herring eggs drying on racks at Ucluelet, 1890s. Royal B.C. Museum photo PN 1176. Prepared for Ucluelet First Nation by Traditions Consulting Services, Inc. Chatwin Engineering Ltd. March 12, 2004 “But the ocean is more the home of these people than the land, and the bounteous gifts of nature in the former element seem more to their taste and are more easily procured than the beasts of the forest.... ...Without a question these people are the richest in every respect in British Columbia...” George Blenkinsop, 1874. Note to Reader Thanks is offered to the Maanulth First Nations for their support of the project for which this is the Final Report, and especially to the h=aw`iih (chiefs), elders and cultural advisors who have shared their knowledge in the past, and throughout the project. In this report, reference is made to “Maanulth First Nations,” a recent term. Within the context of this report, that term is intended to refer to the Huuayaht First Nation, the Uchucklesaht Tribe, the Toquaht First Nation, the Ucluelet First Nation, the Ka:'yu:k't'h/Che:k'tles7et'h' First Nation, and to the tribes and groups that were their predecessors. No attempt has been made to standardize the linguistic transcription of native names or words in this report. These are presented in the manner in which they were encountered in various source materials. Management Summary This is the Final Report for the Culture and Heritage Study, Marine Resource Sites and Activities, Maanulth First Nations. -

Status and Distribution of Marine Birds and Mammals in the Southern Gulf Islands, British Columbia
Status and Distribution of Marine Birds and Mammals in the Southern Gulf Islands, British Columbia. Pete Davidson∗, Robert W Butler∗+, Andrew Couturier∗, Sandra Marquez∗ & Denis LePage∗ Final report to Parks Canada by ∗Bird Studies Canada and the +Pacific WildLife Foundation December 2010 Recommended citation: Davidson, P., R.W. Butler, A. Couturier, S. Marquez and D. Lepage. 2010. Status and Distribution of Birds and Mammals in the Southern Gulf Islands, British Columbia. Bird Studies Canada & Pacific Wildlife Foundation unpublished report to Parks Canada. The data from this survey are publicly available for download at www.naturecounts.ca Bird Studies Canada British Columbia Program, Pacific Wildlife Research Centre, 5421 Robertson Road, Delta British Columbia, V4K 3N2. Canada. www.birdscanada.org Pacific Wildlife Foundation, Reed Point Marine Education Centre, Reed Point Marina, 850 Barnet Highway, Port Moody, British Columbia, V3H 1V6. Canada. www.pwlf.org Contents Executive Summary…………………..……………………………………………………………………………………………1 1. Introduction 1.1 Background and Context……………………………………………………………………………………………………..2 1.2 Previous Studies…………………………………………………………………………………………………………………..5 2. Study Area and Methods 2.1 Study Area……………………………………………………………………………………………………………………………6 2.2 Transect route……………………………………………………………………………………………………………………..7 2.3 Kernel and Cluster Mapping Techniques……………………………………………………………………………..7 2.3.1 Kernel Analysis……………………………………………………………………………………………………………8 2.3.2 Clustering Analysis………………………………………………………………………………………………………8 2.4 -

Victoria Canoe & Kayak Club Newsletter VCKC BBQ September
Victoria Canoe & Kayak Club Newsletter Victoria Canoe & Kayak Club 355 Gorge Road West Victoria, B.C. V9A 1M9 September 2008 Phone: 250-590-8193 (Info only) Website: www.vckc.ca VCKC BBQ September 2, 2008 6:00pm at the Clubhouse It’s the usual potluck with surnames from A-M bringing salads or side dishes and surnames from N-Z bringing desserts . Everyone brings something to BBQ for themselves Beverages are B Y O . VCKC – Newsletter – September 2008 Page 1 Clubhouse News SOOO…! A REMINDER! The club DOES expect PFDs to be worn. A PFD needs to fit YOU, support YOUR September BBQ weight, be done up, and WORN for it to do its job This is for your safety but perhaps even more Don't forget our September BBQ at the General important, for the safety of others who may be Meeting Tuesday, September 2 at 6:00pm. Come and involved in a capsize or rescue with you. One can catch up with everyone’s summer paddling news! drown oneself but not if it endangers others!! The summer has whizzed by as usual. I hope you have RE: Club PFDs all enjoyed it and had a lot of paddling time. It is important to have a PFD that is very comfortable Alan and I haven’t done much paddling so far, as we so that one wears it! The club provides some PFDs were away for three weeks visiting family and friends for the use of beginners so that they can take a overseas and are now enjoying showing B.C. -

West Coast Trail
Hobiton Pacific Rim National Park Reserve Entrance Anchorage Squalicum 60 LEGEND Sachsa TSL TSUNAMI HAZARD ZONE The story behind the trail: Lake Ferry to Lake Port Alberni 14 highway 570 WEST COAST TRAIL 30 60 paved road Sachawil The Huu-ay-aht, Ditidaht and Pacheedaht First Nations However, after the wreck 420 The West Coast Trail (WCT) is one of the three units 30 Self Pt 210 120 30 TSL 30 Lake Aguilar Pt Port Désiré Pachena logging road The Valencia of Pacific Rim National Park Reserve (PRNPR), Helby Is have always lived along Vancouver Island's west coast. of the Valencia in 1906, West Coast Trail forest route Tsusiat IN CASE OF EARTHQUAKE, GO Hobiton administered by Parks Canada. PRNPR protects and km distance in km from Pachena Access TO HIGH GROUND OR INLAND These nations used trails and paddling routes for trade with the loss of 133 lives, 24 300 presents the coastal temperate rainforest, near shore Calamity and travel long before foreign sailing ships reached this the public demanded Cape Beale/Keeha Trail route Creek Mackenzie Bamfield Inlet Lake km waters and cultural heritage of Vancouver Island’s West Coast Bamfield River 2 distance in km from parking lot Anchorage region over 200 the government do River Channel west coast as part of Canada’s national park system. West Coast Trail - beach route outhouse years ago. Over the more to help mariners 120 access century following along this coastline. Trail Map IR 12 Indian Reserve WEST COAST TRAIL POLICY AND PROCEDURES Dianna Brady beach access contact sailors In response the 90 30 The WCT is open from May 1 to September 30. -

British Columbia Regional Guide Cat
National Marine Weather Guide British Columbia Regional Guide Cat. No. En56-240/3-2015E-PDF 978-1-100-25953-6 Terms of Usage Information contained in this publication or product may be reproduced, in part or in whole, and by any means, for personal or public non-commercial purposes, without charge or further permission, unless otherwise specified. You are asked to: • Exercise due diligence in ensuring the accuracy of the materials reproduced; • Indicate both the complete title of the materials reproduced, as well as the author organization; and • Indicate that the reproduction is a copy of an official work that is published by the Government of Canada and that the reproduction has not been produced in affiliation with or with the endorsement of the Government of Canada. Commercial reproduction and distribution is prohibited except with written permission from the author. For more information, please contact Environment Canada’s Inquiry Centre at 1-800-668-6767 (in Canada only) or 819-997-2800 or email to [email protected]. Disclaimer: Her Majesty is not responsible for the accuracy or completeness of the information contained in the reproduced material. Her Majesty shall at all times be indemnified and held harmless against any and all claims whatsoever arising out of negligence or other fault in the use of the information contained in this publication or product. Photo credits Cover Left: Chris Gibbons Cover Center: Chris Gibbons Cover Right: Ed Goski Page I: Ed Goski Page II: top left - Chris Gibbons, top right - Matt MacDonald, bottom - André Besson Page VI: Chris Gibbons Page 1: Chris Gibbons Page 5: Lisa West Page 8: Matt MacDonald Page 13: André Besson Page 15: Chris Gibbons Page 42: Lisa West Page 49: Chris Gibbons Page 119: Lisa West Page 138: Matt MacDonald Page 142: Matt MacDonald Acknowledgments Without the works of Owen Lange, this chapter would not have been possible. -
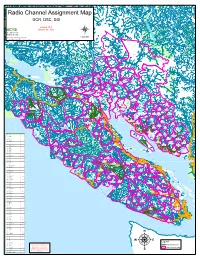
Bcts Dcr, Dsc
Radio Channel Assignment Map DCR, DSC, DSI Version 10.8 BCTS January 30, 2015 BC Timber Sales W a d d i Strait of Georgia n g t o n G l a 1:400,000 c Date Saved: 2/3/2015 9:55:56 AM i S e c r a r Path: F:\tsg_root\GIS_Workspace\Mike\Radio_Frequency\Radio Frequency_2015.mxd C r e e k KLATTASINE BARB HO WARD A A T H K O l MTN H O M l LANDMAR K a i r r C e t l e a n R CAMBRIDG E t e R Wh i E C V A r I W R K A 7 HIDD EN W E J I C E F I E L D Homathko r C A IE R HEAK E T STANTON PLATEAU A G w r H B T TEAQ UAHAN U O S H N A UA Q A E 8 T H B R O I M Southgate S H T N O K A P CUMSACK O H GALLEO N GUNS IGHT R A E AQ V R E I T R r R I C V E R R MT E H V a RALEIG H SAWT rb S tan I t R o l R A u e E HO USE r o B R i y l I l V B E S E i 4 s i R h t h o 17 S p r O G a c l e Bear U FA LCO N T H G A Stafford R T E R E V D I I R c R SMIT H O e PEAK F Bear a KETA B l l F A T SIR FRANCIS DRAKE C S r MT 2 ke E LILLO OE T La P L rd P fo A af St Mellersh Creek PEAKS TO LO r R GRANITE C E T ST J OHN V MTN I I V E R R 12 R TAHUMMING R E F P i A l R Bute East PORTAL E e A L D S R r A E D PEAK O O A S I F R O T T Glendale 11 R T PRATT S N O N 3 S E O M P Phillip I I T Apple River T O L A B L R T H A I I SIRE NIA E U H V 11 ke Po M L i P E La so M K n C ne C R I N re N L w r ro ek G t B I OSMINGTO N I e e Call Inlet m 28 R l o r T e n T E I I k C Orford V R E l 18 V E a l 31 Toba I R C L R Fullmore 5 HEYDON R h R o George 30 Orford River I Burnt Mtn 16 I M V 12 V MATILPI Browne E GEORGE RIVER E R Bute West R H Brem 13 ke Bute East La G 26 don ey m H r l l e U R -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

3LMANUSCRIPT REPORT SERIES No. 36
DFO - L bra y MPOBibio heque II 1 111111 11 11 11 V I 1 120235441 3LMANUSCRIPT REPORT SERIES No. 36 Some If:eat/viz& 3,5,unamia, Olt the Yacific ettadt of South and ✓ cuith anwitica, T. S. Murty, S. 0. Wigen and R. Chawla Marine Sciences Directorate 975 Department of the Environment, Ottawa Marine Sciences Directorate Manuscript. Report Series No. 36 SOME FEATURES OF TSUNAMIS ON THE PACIFIC COAST OF SOUTH AND NORTH AM ERICA . 5 . Molly S . O. Wigen and R. Chawla 1975 Published by Publie par Environment Environnement Canada Canada I' Fisheries and Service des !Aches Marine Service et des sciences de la mer Office of the Editor Bureau du fiedacteur 116 Lisgar, Ottawa K1 A Of13 1 Preface This paper is to be published in Spanish in the Proceedings of the Tsunami Committee XVII Meeting, Lima, Peru 20-31 Aug. 1973, under the International Association of Seismology and Physics of the Earth Interior. 2 Table of Contents Page Abstract - Resume 5 1. Introduction 7 2. Resonance characteristics of sonic inlets on the Pacific Coast of Soulh and North America 13 3. Secondary undulations 25 4. Tsunami forerunner 33 5. Initial withdrawal of water 33 6. Conclusions 35 7. References 37 3 4 i Abstract In order to investigate the response of inlets to tsunamis, the resonance characteristics of some inlets on the coast of Chile have been deduced through simple analytical considerations. A comparison is made with the inlets of southeast Alaska, the mainland coast of British Columbia and Vancouver Island. It is shown that the general level of intensif yy of secondary undulations is highest for Vancouver Island inlets, and least for those of Chile and Alaska.