Exercise-Induced Bronchoconstriction and Asthma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Differentiating Between Anxiety, Syncope & Anaphylaxis
Differentiating between anxiety, syncope & anaphylaxis Dr. Réka Gustafson Medical Health Officer Vancouver Coastal Health Introduction Anaphylaxis is a rare but much feared side-effect of vaccination. Most vaccine providers will never see a case of true anaphylaxis due to vaccination, but need to be prepared to diagnose and respond to this medical emergency. Since anaphylaxis is so rare, most of us rely on guidelines to assist us in assessment and response. Due to the highly variable presentation, and absence of clinical trials, guidelines are by necessity often vague and very conservative. Guidelines are no substitute for good clinical judgment. Anaphylaxis Guidelines • “Anaphylaxis is a potentially life-threatening IgE mediated allergic reaction” – How many people die or have died from anaphylaxis after immunization? Can we predict who is likely to die from anaphylaxis? • “Anaphylaxis is one of the rarer events reported in the post-marketing surveillance” – How rare? Will I or my colleagues ever see a case? • “Changes develop over several minutes” – What is “several”? 1, 2, 10, 20 minutes? • “Even when there are mild symptoms initially, there is a potential for progression to a severe and even irreversible outcome” – Do I park my clinical judgment at the door? What do I look for in my clinical assessment? • “Fatalities during anaphylaxis usually result from delayed administration of epinephrine and from severe cardiac and respiratory complications. “ – What is delayed? How much time do I have? What is anaphylaxis? •an acute, potentially -

Dosing Time Matters
bioRxiv preprint doi: https://doi.org/10.1101/570119; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Dosing Time Matters 1 2,3 4,5,6 1* Marc D. Ruben , David F. Smith , Garret A. FitzGerald , and John B. Hogenesch 1 Division of Human Genetics, Center for Chronobiology, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, 240 Albert Sabin Way, Cincinnati, OH, 45229 2 Divisions of Pediatric Otolaryngology and Pulmonary and Sleep Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229 3 Department of Otolaryngology-Head and Neck Surgery, University of Cincinnati School of Medicine, 231 Albert Sabin Way, Cincinnati, OH, 45267 4 Department of Systems Pharmacology and Translational Therapeutics, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 5 Department of Medicine, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 6 Institute for Translational Medicine and Therapeutics (ITMAT), at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA *Corresponding Author. Email: [email protected] Abstract Trainees in medicine are taught to diagnose and administer treatment as needed; time-of-day is rarely considered. Yet accumulating evidence shows that ~half of human genes and physiologic functions follow daily rhythms. Circadian medicine aims to incorporate knowledge of these rhythms to enhance diagnosis and treatment. -

(Loxapine) REMS
Current as of 6/1/2013.® This document may not be part of the latest approved REMS. Alexza Pharmaceuticals, Inc. 2091 Stierlin Court, Mountain View, CA 94043 IMPORTANT DRUG WARNING Risk of bronchospasm with ADASUVE™ (loxapine) inhalation powder ADASUVE™ available only in enrolled healthcare facilities, under an FDA-required REMS Program Dear Healthcare Professional: This letter contains important safety information for ADASUVE™ (loxapine) Inhalation Powder. ADASUVE is a drug-device combination product that delivers the antipsychotic, loxapine, by oral inhalation. ADASUVE has been approved by the US Food and Drug Administration (FDA) for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults. FDA has determined that a Risk Evaluation and Mitigation Strategy (REMS) is necessary to ensure that the benefits of treatment outweigh the risk of bronchospasm in patients treated with ADASUVE. ADASUVE is not available in an outpatient setting. If you are an outpatient provider, you are receiving this letter because ADASUVE may be, or may have been, administered to one of your patients in an enrolled healthcare facility. Enrolled healthcare facilities, such as an acute care or inpatient setting, must have immediate on-site access to equipment and personnel trained to manage acute bronchospasm, including advanced airway management, eg, intubation and mechanical ventilation. (See below for more facility enrollment requirements.) It is important that anyone caring for patients treated with ADASUVE be aware of the risk of bronchospasm after administration. This letter does not contain a complete list of all the risks associated with ADASUVE. Reference ID:Please 3235446 see the enclosed full prescribing information for more information. -

Maximum Expiratory Flow Rates in Induced Bronchoconstriction in Man
Maximum expiratory flow rates in induced bronchoconstriction in man A. Bouhuys, … , B. M. Kim, A. Zapletal J Clin Invest. 1969;48(6):1159-1168. https://doi.org/10.1172/JCI106073. Research Article We evaluated changes of maximum expiratory flow-volume (MEFV) curves and of partial expiratory flow-volume (PEFV) curves caused by bronchoconstrictor drugs and dust, and compared these to the reverse changes induced by a bronchodilator drug in previously bronchoconstricted subjects. Measurements of maximum flow at constant lung inflation (i.e. liters thoracic gas volume) showed larger changes, both after constriction and after dilation, than measurements of peak expiratory flow rate, 1 sec forced expiratory volume and the slope of the effort-independent portion of MEFV curves. Changes of flow rates on PEFV curves (made after inspiration to mid-vital capacity) were usually larger than those of flow rates on MEFV curves (made after inspiration to total lung capacity). The decreased maximum flow rates after constrictor agents are not caused by changes in lung static recoil force and are attributed to narrowing of small airways, i.e., airways which are uncompressed during forced expirations. Changes of maximum expiratory flow rates at constant lung inflation (e.g. 60% of the control total lung capacity) provide an objective and sensitive measurement of changes in airway caliber which remains valid if total lung capacity is altered during treatment. Find the latest version: https://jci.me/106073/pdf Maximum Expiratory Flow Rates in Induced Bronchoconstriction in Man A. Bouiuys, V. R. HuNTr, B. M. Kim, and A. ZAPLETAL From the John B. Pierce Foundation Laboratory and the Yale University School of Medicine, New Haven, Connecticut 06510 A B S T R A C T We evaluated changes of maximum ex- rates are best studied as a function of lung volume. -

Ep 2560611 B1
(19) TZZ Z___T (11) EP 2 560 611 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/00 (2006.01) 03.01.2018 Bulletin 2018/01 (86) International application number: (21) Application number: 11719211.2 PCT/EP2011/056227 (22) Date of filing: 19.04.2011 (87) International publication number: WO 2011/131663 (27.10.2011 Gazette 2011/43) (54) "PROCESS FOR PROVIDING PARTICLES WITH REDUCED ELECTROSTATIC CHARGES" VERFAHREN ZUR BEREITSTELLUNG VON PARTIKELN MIT REDUZIERTEN ELEKTROSTATISCHEN LADUNGEN PROCÉDÉ DE PRÉPARATION DE PARTICULES AYANT DES CHARGES ÉLECTROSTATIQUES RÉDUITES (84) Designated Contracting States: • GUCHARDI ET AL: "Influence of fine lactose and AL AT BE BG CH CY CZ DE DK EE ES FI FR GB magnesium stearate on low dose dry powder GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO inhaler formulations", INTERNATIONAL PL PT RO RS SE SI SK SM TR JOURNAL OF PHARMACEUTICS, ELSEVIER BV, NL LNKD- DOI:10.1016/J.IJPHARM.2007.06.041, (30) Priority: 21.04.2010 EP 10160565 vol. 348, no. 1-2, 19 December 2007 (2007-12-19), pages 10-17, XP022393884, ISSN: 0378-5173 (43) Date of publication of application: • ELAJNAF A ET AL: "Electrostatic 27.02.2013 Bulletin 2013/09 characterisation of inhaled powders: Effect of contact surface and relative humidity", (73) Proprietor: Chiesi Farmaceutici S.p.A. EUROPEAN JOURNAL OF PHARMACEUTICAL 43100 Parma (IT) SCIENCES, ELSEVIER, AMSTERDAM, NL LNKD- DOI:10.1016/J.EJPS.2006.07.006, vol. 29, no. 5, 1 (72) Inventors: December 2006 (2006-12-01), pages 375-384, • COCCONI, Daniela XP025137181, ISSN: 0928-0987 [retrieved on I-43100 Parma (IT) 2006-12-01] • MUSA, Rossella • CHAN ET AL: "Dry powder aerosol drug I-43100 Parma (IT) delivery-Opportunities for colloid and surface scientists", COLLOIDS AND SURFACES. -

Bronchoconstriction in Normal and Asthmatic Subjects
Thorax: first published as 10.1136/thx.43.11.890 on 1 November 1988. Downloaded from Thorax 1988;43:890-895 The nasal response to exercise and exercise induced bronchoconstriction in normal and asthmatic subjects KINGMAN P STROHL, MICHAEL J DECKER, LESLIE G OLSON, TOD A FLAK, PETER L HOEKJE Airway Disease Center, Departments ofMedicine, University Hospitals ofCleveland; and Case Western Reserve University, Cleveland, Ohio, USA ABSTRACT Two studies were carried out to test the hypothesis that the fall and recovery of nasal resistance after exercise in asthmatic and non-asthmatic subjects are related to the development of bronchoconstriction after exercise. In study 1 nasal resistance (posterior rhinomanometry) and specific airway resistance (sRaw) were measured before challenge and one, five, 10 and 30 minutes after four minutes of exhausting legwork exercise in nine asthmatic subjects and nine age matched healthy subjects. One minute after exercise there was a reduction in nasal resistance of49% (SD 15%) from baseline in the healthy subjects and of 66% (17%) in the asthmatic subjects. This response and the subsequent return ofnasal resistance to baseline values did not differ significantly between the two groups despite a substantial difference in the change in sRaw, an increase of 74% (45%) in the asthmatic subjects 10 minutes after exercise, and no change in the non-asthmatic subjects. In study 2, nasal and specific airway resistances were monitored according to the same measurement protocolcopyright. in six subjects with increased airway reactivity. Subjects exercised on two occasions, wearing a noseclip, once while breathing cold, dry air and once while breathing warm, humid air. -

Inhaled Loxapine Monograph
Inhaled Loxapine Monograph Inhaled Loxapine (ADASUVE) National Drug Monograph February 2015 VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives The purpose of VA PBM Services drug monographs is to provide a comprehensive drug review for making formulary decisions. Updates will be made when new clinical data warrant additional formulary discussion. Documents will be placed in the Archive section when the information is deemed to be no longer current. FDA Approval Information Description/Mechanism of Inhaled loxapine is a typical antipsychotic used in the treatment of acute Action agitation associated with schizophrenia and bipolar I disorder in adults. Loxapine’s mechanism of action for reducing agitation in schizophrenia and bipolar I disorder is unknown. Its effects are thought to be mediated through blocking postsynaptic dopamine D2 receptors as well as some activity at the serotonin 5-HT2A receptors. Indication(s) Under Review in Inhaled loxapine is a typical antipsychotic indicated for the acute treatment of this document (may include agitation associated with schizophrenia or bipolar I disorder in adults. off label) Off-label use Agitation related to any other cause not due to schizophrenia and bipolar I disorder. Dosage Form(s) Under 10mg oral inhalation using a new STACCATO inhaler device. Review REMS REMS No REMS Post-marketing Study Required See Other Considerations for additional REMS information Pregnancy Rating C Executive Summary Efficacy Inhaled loxapine was superior to placebo in reducing acute agitation at 2 hours post dose measured by the Positive and Negative Syndrome Scale-Excited Component (PEC) in patients with bipolar I disorder and schizophrenia. -
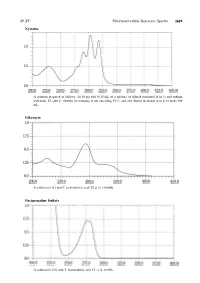
Nystatin Ofloxacin Orciprenaline Sulfate
JP XV Ultraviolet-visible Reference Spectra 1619 Nystatin A solution prepared as follows: To 10 mg add 50.25 mL of a mixture of diluted methanol (4 in 5) and sodium hydroxide TS (200:1), dissolve by warming at not exceeding 509C,andadddilutedmethanol(4in5)tomake500 mL. Ofloxacin Asolutionin0.1mol/LhydrochloricacidTS(1in150,000) Orciprenaline Sulfate A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) 1620 Ultraviolet-visible Reference Spectra JP XV Oxazolam A solution in ethanol (95) (1 in 100,000) Oxethazaine A solution in ethanol (95) (1 in 2500) Oxybuprocaine Hydrochloride An aqueous solution (1 in 100,000) JP XV Ultraviolet-visible Reference Spectra 1621 Oxycodone Hydrochloride Hydrate An aqueous solution (1 in 10,000) Oxymetholone A solution prepared as follows: To 5 mL of a solution in methanol (1 in 5000) add 5 mL of sodium hydroxide-methanol TS and methanol to make 50 mL. Oxytetracycline Hydrochloride Asolutionin0.1mol/LhydrochloricacidTS(1in50,000) 1622 Ultraviolet-visible Reference Spectra JP XV Oxytocin An aqueous solution (1 in 2000) Penbutolol Sulfate A solution in methanol (1 in 10,000) Pentazocine A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) JP XV Ultraviolet-visible Reference Spectra 1623 Peplomycin Sulfate Asolutionpreparedasfollows:To4mgadd5mL of copper (II) sulfate TS, and dissolve in water to make 100 mL. Perphenazine 1 Asolutionin0.1mol/LhydrochloricacidTS(1in200,000) Perphenazine 2 A solution obtained by adding 10 mL of water to 10 mL of the solution for Perphenazine 1 1624 Ultraviolet-visible -

Β2 Adrenergic Agonist Suppresses Eosinophil-Induced Epithelial-To- Mesenchymal Transition of Bronchial Epithelial Cells Keigo Kainuma1,2, Tetsu Kobayashi3, Corina N
Kainuma et al. Respiratory Research (2017) 18:79 DOI 10.1186/s12931-017-0563-4 RESEARCH Open Access β2 adrenergic agonist suppresses eosinophil-induced epithelial-to- mesenchymal transition of bronchial epithelial cells Keigo Kainuma1,2, Tetsu Kobayashi3, Corina N. D’Alessandro-Gabazza2, Masaaki Toda2, Taro Yasuma2, Kota Nishihama2, Hajime Fujimoto3, Yu Kuwabara1,2, Koa Hosoki1,2, Mizuho Nagao1, Takao Fujisawa1 and Esteban C. Gabazza2* Abstract Background: Epithelial-mesenchymal transition is currently recognized as an important mechanism for the increased number of myofibroblasts in cancer and fibrotic diseases. We have already reported that epithelial- mesenchymal transition is involved in airway remodeling induced by eosinophils. Procaterol is a selective and full β2 adrenergic agonist that is used as a rescue of asthmatic attack inhaler form and orally as a controller. In this study, we evaluated whether procaterol can suppress epithelial-mesenchymal transition of airway epithelial cells induced by eosinophils. Methods: Epithelial-mesenchymal transition was assessed using a co-culture system of human bronchial epithelial cells and primary human eosinophils or an eosinophilic leukemia cell line. Results: Procaterol significantly inhibited co-culture associated morphological changes of bronchial epithelial cells, decreased the expression of vimentin, and increased the expression of E-cadherin compared to control. Butoxamine, a specific β2-adrenergic antagonist, significantly blocked changes induced by procaterol. In addition, procaterol inhibited the expression of adhesion molecules induced during the interaction between eosinophils and bronchial epithelial cells, suggesting the involvement of adhesion molecules in the process of epithelial-mesenchymal transition. Forskolin, a cyclic adenosine monophosphate-promoting agent, exhibits similar inhibitory activity of procaterol. Conclusions: Overall, these observations support the beneficial effect of procaterol on airway remodeling frequently associated with chronic obstructive pulmonary diseases. -
Ep 2626065 A1
(19) TZZ Z_T (11) EP 2 626 065 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: A61K 31/137 (2006.01) A61K 31/135 (2006.01) 14.08.2013 Bulletin 2013/33 A61K 31/4704 (2006.01) A61K 31/58 (2006.01) A61K 31/56 (2006.01) A61K 9/12 (2006.01) (2006.01) (2006.01) (21) Application number: 11827927.2 A61K 9/14 A61P 11/06 (86) International application number: Date of filing: 01.02.2011 (22) PCT/CN2011/070883 (87) International publication number: WO 2012/041031 (05.04.2012 Gazette 2012/14) (84) Designated Contracting States: (72) Inventor: WU, Wei-hsiu AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Taipei GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Taiwan (TW) PL PT RO RS SE SI SK SM TR (74) Representative: Patentanwaltskanzlei WILHELM (30) Priority: 28.09.2010 CN 201010502339 & BECK Prinzenstrasse 13 (71) Applicant: Intech Biopharm Ltd. 80639 München (DE) Taipei (TW) (54) COMPOUND COMPOSITION FOR INHALATION USED FOR TREATING ASTHMA (57) An inhaled pharmaceutical composition con- tric way as a controller. The eccentric way control therapy tains primary active ingredients of beta2- agonist and cor- could create a low blood concentration period during the ticosteroids.The pharmaceuticalcompositions disclosed day and minimize the acute tolerance phenomenon (or in the present invention are to be inhaled by a patient so called tachyphylaxis) for bronchodilator - beta2-ago- when needed as a reliever, or administrated in an eccen- nists in treating asthma or other obstructive respiratory disorders. -

Clenbuterol Elisa Kit Instructions Product #101219 & 101216 Forensic Use Only
Neogen Corporation 944 Nandino Blvd., Lexington KY 40511 USA 800/477-8201 USA/Canada | 859/254-1221 Fax: 859/255-5532 | E-mail: [email protected] | Web: www.neogen.com/Toxicology CLENBUTEROL ELISA KIT INSTRUCTIONS PRODUCT #101219 & 101216 FORENSIC USE ONLY INTENDED USE: For the determination of trace quantities of Clenbuterol and/or other metabolites in human urine, blood, oral fluid. DESCRIPTION Neogen Corporation’s Clenbuterol ELISA (Enzyme-Linked ImmunoSorbent Assay) test kit is a qualitative one-step kit designed for use as a screening device for the detection of drugs and/or their metabolites. The kit was designed for screening purposes and is intended for forensic use only. It is recommended that all suspect samples be confirmed by a quantitative method such as gas chromatography/mass spectrometry (GC/MS). ASSAY PRINCIPLES Neogen Corporation’s test kit operates on the basis of competition between the drug or its metabolite in the sample and the drug- enzyme conjugate for a limited number of antibody binding sites. First, the sample or control is added to the microplate. Next, the diluted drug-enzyme conjugate is added and the mixture is incubated at room temperature. During this incubation, the drug in the sample or the drug-enzyme conjugate binds to antibody immobilized in the microplate wells. After incubation, the plate is washed 3 times to remove any unbound sample or drug-enzyme conjugate. The presence of bound drug-enzyme conjugate is recognized by the addition of K-Blue® Substrate (TMB). After a 30 minute substrate incubation, the reaction is halted with the addition of Red Stop Solution. -

Exercise Induced Bronchoconstriction (EIB) What Is Exercise Induced Bronchoconstriction Testing?
Exercise Induced Bronchoconstriction (EIB) What is Exercise Induced Bronchoconstriction testing? Exercise induced bronchoconstriction or EIB, is a combined breathing and exercise test. The test can help identify what type of breathing trouble you have, if any, when you exercise. A spirometry breathing test is done before and after you exercise on a treadmill. Spirometry can show how much air you can breathe in and out. It also shows how fast you can breathe in and out. The spirometry results are compared before and after you exercise to see what changes there are in your breathing. A laryngoscopy may be scheduled after the EIB test. A laryngoscopy is often done to identify if your vocal cords may be causing you to have trouble breathing with exercise. How do you get ready for the test? Please follow these directions when getting ready for this test. These medicines will affect the results of some of these tests and may need to be stopped before the testing is done. If the medicine is not stopped, as your doctor says, before the test we will not be able to complete the test. • Stop these inhaled medicines for 48 hours before your appointment: ◦ Anora® (umeclidinium and vilanterol) ◦ Bevespi® (glycopyrrolate and formoterol) ◦ Stiolto® (olodaterol and tiotropium) ◦ Utibron® (indacaterol and glycopyrrolate) ◦ Trelegy® (fluticasone, umeclidinium and vilanterol) • Stop these inhaled medicines for 24 hours before your appointment: ◦ Incruse® (umeclidinium) ◦ Seebri® (glycopyrrolate) ◦ Spiriva® (tiotropium) ◦ Tudorza® (aclidinium) • Stop these