Lethal Yellowing (LY) of Palm1 Brian W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aiphanes Grandis Borchs
Palm Conservation – Palm Specialist Group Aiphanes grandis Borchs. & Balslev Status: Endangered (EN) Common name None recorded Natural range Aiphanes grandis is endemic to the western slopes of the Andes mountains in Ecuador, at elevations between 1000 and 2000 m. It occurs in cloud forest in the central and southern parts of the country. Recognition characteristics It is a large, solitary palm tree, with a stem up to 20 m tall and 10–20 cm in diameter. The stem and leaves are fiercely armed with long, black spines. The leaf blade is 200–250 cm long with up to 50 leaflets on each side, briefly jagged at apex, inserted in groups and pointing in different directions. The inflorescence is 1–1.5 m long, with up to 200 spreading branches. Flowers are white to pale yellow and fruits 2–3 cm in diameter, dull green and covered with brown, loosely attached bristles. The combination of jagged leaflets and large size distinguishes the species from all other South American palms. Natural history Little is known about the natural history of the species. The palm heart is edible, and its seeds are sometimes pureed and cooked with crude cane sugar to form a nougat-like paste. Threats to survival The estimated loss of potential habitat for A. grandis, based on habitat modeling, is 62%, most of which has occurred over the last 30–40 years. The west Andean mountain forest in southern and central Ecudor, where A. grandis occurs, harbors a high number of endemic plant species. It is also the home to remnant populations of such conspicuous palm species as Ceroxylon ventricosum and C. -

Thrinax Radiata Family: Arecaceae Florida Thatch Palm, Jamaican Thatch, Thatch Palm, Chit
Stephen H. Brown, Horticulture Agent Donna Cressman, Master Gardener Lee County Extension, Fort Myers, Florida (239) 533-7513 [email protected] http://lee.ifas.ufl.edu/hort/GardenHome.shtml Thrinax radiata Family: Arecaceae Florida thatch palm, Jamaican thatch, thatch palm, chit Florida Thatch Palm Synonyms (Discarded names): Cocothrinax martii, C. radiate, Thrinax floridana, T. martii, T. multiflora; T. wendlandiana Origin: Extreme southern mainland coast of Florida, Florida Keys, Bahamas, western Cuba, Cayman Islands, Jamaica, Hispaniola, Puerto Rico, Yucatan Peninsula, Honduras, Nicaragua U.S.D.A. Zone: 10A-12B (28°F leaf damage) Growth Rate: Slow Typical Height: 20’ Habit: Solitary; canopy of 12-20 leaves Crownshaft: None Leaf: Palmate, induplicate, circular, slightly folded; divided about halfway into segments that are split at the tips; pointed hastula Leaf Size: 4-5’ wide; segments 2.5’ long, 2” wide Salt Tolerance: High Drought Tolerance: High Wind Tolerance: High Light Requirements: Moderate, high Soil: Widely adaptable Nutritional Requirements: Low Potential Insect Pests: Aphids; scales Propagation: Seeds Human hazards: None Uses: Small gardens; containers; outdoors patios; roadways; parking lots; seasides; specimen Left: The infructescence (fruited stems) hang in a circle around the trunk, sometimes extending beyond the leaf. Natural Geographic Distribution The Florida Thatch Palm, Thrinax radiata, is indigenous to the extreme southern mainland coast of Florida, the Florida Keys, Bahamas, western Cuba, The Cayman Islands, Jamaica, Hispaniola, Puerto Rico, Honduras, Nicaragua, and the eastern coast of the Yucatan Peninsula in Mexico and Belize. In na- ture, this palm almost always grows within the range of salt-laden winds near coastal areas. It grows naturally in sandy or calcareous soils. -

TAXON:Phoenix Sylvestris SCORE:5.0 RATING:Evaluate
TAXON: Phoenix sylvestris SCORE: 5.0 RATING: Evaluate Taxon: Phoenix sylvestris Family: Arecaceae Common Name(s): date sugar palm Synonym(s): Elate sylvestris L. (basionym) Indian date silver date palm wild date palm Assessor: No Assessor Status: Assessor Approved End Date: 29 Jul 2014 WRA Score: 5.0 Designation: EVALUATE Rating: Evaluate Keywords: Naturalized, Tropical Palm, Spiny, Dioecious, Bird-dispersed Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 n Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 y outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 y 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) n 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed n=0, y = 2*multiplier (see Appendix 2) n 305 Congeneric weed n=0, y = 1*multiplier (see Appendix 2) y 401 Produces spines, thorns or burrs -

Arizona Landscape Palms
Cooperative Extension ARIZONA LANDSCAPE PALMS ELIZABETH D AVISON Department of Plant Sciences JOHN BEGEMAN Pima County Cooperative Extension AZ1021 • 12/2000 Issued in furtherance of Cooperative Extension work acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, James A. Christenson, Director, Cooperative Extension, College of Agriculture and Life Sciences, The University of Arizona. The University of Arizona College of Agriculture and Life Sciences is an equal opportunity employer authorized to provide research, educational information and other services to individuals and institutions that function without regard to sex, race, religion, color, national origin, age, Vietnam Era Veteran's status, or disability. Contents Landscape Use ......................................... 3 Adaptation ................................................ 3 Planting Palms ......................................... 3 Care of Established Palms...................... 5 Diseases and Insect Pests ....................... 6 Palms for Arizona .................................... 6 Feather Palms ........................................... 8 Fan Palms................................................ 12 Palm-like Plants ..................................... 16 This information has been reviewed by university faculty. ag.arizona.edu/pubs/garden/az1121.pdf 2 The luxuriant tropical appearance and stately Adaptation silhouette of palms add much to the Arizona landscape. Palms generally can be grown below the 4000 ft level Few other plants are as striking in low and mid elevation in Arizona. However, microclimate may make the gardens. Although winter frosts and low humidity limit difference between success and failure in a given location. the choices somewhat, a good number of palms are Frost pockets, where nighttime cold air tends to collect, available, ranging from the dwarf Mediterranean Fan should be avoided, especially for the tender species. Palms palm to the massive Canary Island Date palm. -
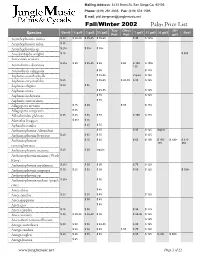
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

The Origin of Monocotyledons from Dicotyledons, Through Self-Adaptation to a Moist
The Origin of Monocotyledons from Dicotyledons, through Self-adaptation to a Moist. or Aquatic Habit.1 BY G. HENSLOW, M.A., F.L.S., F.G.S., F.R.H.S., V.M.H. CONTENTS. SECTIONS. i PAGE 1. INTRODUCTION . 717 2. Evidences from Geology ........... 718 3. Distribution and Percentages of the Natural Orders of Monocotyledons ns com- pared with those of Dicotyledons . 719 4. Degeneracy of Monocotyledons 720 5. Possible Aquatic Origin.of Palms 723 6. Leaves of Large Size characteristic of many Aquatic Plants .... 734 7. Water-storage Organs ........... 724 8. The Requirement of much Water by many Terrestrial Monocotyledons . 724 9. Cycads and Monocotyledons . 725 10. Monocotyledonous Dicotyledons . • 727 11. The Effects of Water upon Roots 731 12. Origin of the formerly called 'Endogenous' Arrangement of the Cauline Bundles of Monocotyledons . ........ 73a 13. The Forms and Structure of Aquatic Leaves are the Result of the Direct Action of Water -735 14. The Reticulated Venation of some Monocotyledons is only imitative of that of Dicotyledons 736 15. Reproductive Organs ............ 737 16. Cytological and Embryological Investigations 738 17. Speculations on the Arrest of one Cotyledon ....... 74° 18. Non-inheritance, Imperfect and Complete Inheritance, of Acquired Characters . 741 19. Isolation and Natural Selection 743 20. CONCLUSION 743 1. INTRODUCTION. EARLY twenty years ago (1892), I pointed out that there is a large N number of coincidences between the morphological and anatomical characters of Monocotyledons and Aquatic Dicotyledons, sufficient, in fact, to justify the conception that the former class had been evolved from the latter. Beyond a limited extent in experiments upon adaptation, the 1 Supplementary Observations and Experiments to ' A Theoretical Origin of Endogens from Exogens, through Self-adaptation to an Aquatic Habit'. -

Trachycarpus Fortunei) and Its Application Potential
Article Anatomy of the Windmill Palm (Trachycarpus fortunei) and Its Application Potential Jiawei Zhu 1, Jing Li 1,2, Chuangui Wang 3 and Hankun Wang 1,* 1 Department of Biomaterials, International Center for Bamboo and Rattan, State Forestry Administration and Beijing Co-Built Key Lab for Bamboo and Rattan Science & Technology, Beijing 100102, China; [email protected] (J.Z.); [email protected] (J.L.) 2 Key Lab of Wood Science and Technology of National Forestry and Grassland Administration, Research Institute of Wood Industry, Chinese Academy of Forestry, Beijing 100102, China 3 School of Forestry & Landscape Architecture, Anhui Agricultural University, Hefei 230036, China; [email protected] * Correspondence: [email protected] Received: 13 November 2019; Accepted: 9 December 2019; Published: 10 December 2019 Abstract: The windmill palm (Trachycarpus fortunei (Hook.) H. Wendl.) is widely distributed and is an important potential source of lignocellulosic materials. The lack of knowledge on the anatomy of the windmill palm has led to its inefficient use. In this paper, the diversity in vascular bundle types, shape, surface, and tissue proportions in the leaf sheaths and stems were studied with digital microscopy and scanning electron microscope (SEM). Simultaneously, fiber dimensions, fiber surfaces, cell wall ultrastructure, and micromechanics were studied with atomic force microscopy (AFM) and a nanoindenter. There is diversity among vascular bundles in stems and leaf sheaths. All vascular bundles in the stems are type B (circular vascular tissue (VT) at the edge of the fibrous sheath (FS)) while the leaf sheath vascular bundles mostly belong to type C (aliform (VT) at the center of the (FS), with the wings of the (VT) extending to the edge of the vascular bundles). -

Monocotyledons and Gymnosperms of Puerto Rico and the Virgin Islands
SMITHSONIAN INSTITUTION Contributions from the United States National Herbarium Volume 52: 1-415 Monocotyledons and Gymnosperms of Puerto Rico and the Virgin Islands Editors Pedro Acevedo-Rodríguez and Mark T. Strong Department of Botany National Museum of Natural History Washington, DC 2005 ABSTRACT Acevedo-Rodríguez, Pedro and Mark T. Strong. Monocots and Gymnosperms of Puerto Rico and the Virgin Islands. Contributions from the United States National Herbarium, volume 52: 415 pages (including 65 figures). The present treatment constitutes an updated revision for the monocotyledon and gymnosperm flora (excluding Orchidaceae and Poaceae) for the biogeographical region of Puerto Rico (including all islets and islands) and the Virgin Islands. With this contribution, we fill the last major gap in the flora of this region, since the dicotyledons have been previously revised. This volume recognizes 33 families, 118 genera, and 349 species of Monocots (excluding the Orchidaceae and Poaceae) and three families, three genera, and six species of gymnosperms. The Poaceae with an estimated 89 genera and 265 species, will be published in a separate volume at a later date. When Ackerman’s (1995) treatment of orchids (65 genera and 145 species) and the Poaceae are added to our account of monocots, the new total rises to 35 families, 272 genera and 759 species. The differences in number from Britton’s and Wilson’s (1926) treatment is attributed to changes in families, generic and species concepts, recent introductions, naturalization of introduced species and cultivars, exclusion of cultivated plants, misdeterminations, and discoveries of new taxa or new distributional records during the last seven decades. -

Ornamental Garden Plants of the Guianas, Part 3
; Fig. 170. Solandra longiflora (Solanaceae). 7. Solanum Linnaeus Annual or perennial, armed or unarmed herbs, shrubs, vines or trees. Leaves alternate, simple or compound, sessile or petiolate. Inflorescence an axillary, extra-axillary or terminal raceme, cyme, corymb or panicle. Flowers regular, or sometimes irregular; calyx (4-) 5 (-10)- toothed; corolla rotate, 5 (-6)-lobed. Stamens 5, exserted; anthers united over the style, dehiscing by 2 apical pores. Fruit a 2-celled berry; seeds numerous, reniform. Key to Species 1. Trees or shrubs; stems armed with spines; leaves simple or lobed, not pinnately compound; inflorescence a raceme 1. S. macranthum 1. Vines; stems unarmed; leaves pinnately compound; inflorescence a panicle 2. S. seaforthianum 1. Solanum macranthum Dunal, Solanorum Generumque Affinium Synopsis 43 (1816). AARDAPPELBOOM (Surinam); POTATO TREE. Shrub or tree to 9 m; stems and leaves spiny, pubescent. Leaves simple, toothed or up to 10-lobed, to 40 cm. Inflorescence a 7- to 12-flowered raceme. Corolla 5- or 6-lobed, bluish-purple, to 6.3 cm wide. Range: Brazil. Grown as an ornamental in Surinam (Ostendorf, 1962). 2. Solanum seaforthianum Andrews, Botanists Repository 8(104): t.504 (1808). POTATO CREEPER. Vine to 6 m, with petiole-tendrils; stems and leaves unarmed, glabrous. Leaves pinnately compound with 3-9 leaflets, to 20 cm. Inflorescence a many- flowered panicle. Corolla 5-lobed, blue, purple or pinkish, to 5 cm wide. Range:South America. Grown as an ornamental in Surinam (Ostendorf, 1962). Sterculiaceae Monoecious, dioecious or polygamous trees and shrubs. Leaves alternate, simple to palmately compound, petiolate. Inflorescence an axillary panicle, raceme, cyme or thyrse. -

Hybridization in the Genus Phoenix: a Review
Emir. J. Food Agric. 2013. 25 (11): 831-842 doi: 10.9755/ejfa.v25i11.16660 http://www.ejfa.info/ REVIEW ARTICLE Hybridization in the genus Phoenix: A review Muriel Gros-Balthazard* University of Fribourg, Department of Biology, Biochemistry, Chemin du Musée 10, 1700 Fribourg, Switzerland Abstract The genus Phoenix is composed of 14 species naturally distributed in the Old World. This genus comprises the date palm, Phoenix dactylifera L., cultivated for its fruits, the dates, while other species are grown for food, ornament and religious purposes. Phoenix species were, for these reasons, spread out of their natural distribution area. It is therefore common to find species not naturally sympatric, growing together, in cultivation or in the wild. Phoenix species are interfertile and crossing distinct species leads to fertile hybrid offspring (interspecific hybridization). The introduction of a species in the wild generates gene flows leading to the creation of new hybrids and has conservation implications. In cultivation, such crossings may be spontaneous or are the result of artificial pollination, as several reasons impel doing so. Crossing gives rise to beautiful hybrids and is also useful for the conservation of old palm groves threatened by pests. Moreover, artificial pollination of date palms using another Phoenix species can be of interest given the metaxenic pollen effects. In addition, this process may have some potential benefits in date palm improvements, by the creation of hybrid cultivars. Thus, an increasing need of hybrid detection and characterization exists, particularly as morphology alone is not sufficient for this task. Besides new methods such as traditional and geometric morphometrics that may bring new clues, the advent of genetic and molecular markers helps to detect hybrids, especially based on the combination of nuclear and chloroplastic data. -

A Review of Animal-Mediated Seed Dispersal of Palms
Selbyana 11: 6-21 A REVIEW OF ANIMAL-MEDIATED SEED DISPERSAL OF PALMS SCOTT ZoNA Rancho Santa Ana Botanic Garden, 1500 North College Avenue, Claremont, California 91711 ANDREW HENDERSON New York Botanical Garden, Bronx, New York 10458 ABSTRACT. Zoochory is a common mode of dispersal in the Arecaceae (palmae), although little is known about how dispersal has influenced the distributions of most palms. A survey of the literature reveals that many kinds of animals feed on palm fruits and disperse palm seeds. These animals include birds, bats, non-flying mammals, reptiles, insects, and fish. Many morphological features of palm infructescences and fruits (e.g., size, accessibility, bony endocarp) have an influence on the animals which exploit palms, although the nature of this influence is poorly understood. Both obligate and opportunistic frugivores are capable of dispersing seeds. There is little evidence for obligate plant-animaI mutualisms in palm seed dispersal ecology. In spite of a considerable body ofliterature on interactions, an overview is presented here ofthe seed dispersal (Guppy, 1906; Ridley, 1930; van diverse assemblages of animals which feed on der Pijl, 1982), the specifics ofzoochory (animal palm fruits along with a brief examination of the mediated seed dispersal) in regard to the palm role fruit and/or infructescence morphology may family have been largely ignored (Uhl & Drans play in dispersal and subsequent distributions. field, 1987). Only Beccari (1877) addressed palm seed dispersal specifically; he concluded that few METHODS animals eat palm fruits although the fruits appear adapted to seed dispersal by animals. Dransfield Data for fruit consumption and seed dispersal (198lb) has concluded that palms, in general, were taken from personal observations and the have a low dispersal ability, while Janzen and literature, much of it not primarily concerned Martin (1982) have considered some palms to with palm seed dispersal. -

Lista De Palmas Cubanas I- Hemithrinax
ISSN 2519-7754 RNPS 2402 www.revistas.geotech.cu/index.php/abc ║LISTA DE ESPECIES║ Vol. 218, No.1 (enero-abril 2019): 1-10 Lista de Palmas Cubanas. I. Hemithrinax, Leucothrinax y Thrinax Cuban Palms Checklist. I. Hemithrinax, Leucothrinax and Thrinax Celio E. Moya López R SU N Autor para correspondencia: [email protected] Se actualiza la lista de táxones y de sinónimos nomenclaturales de los géneros Hemithrinax, Leucothrinax y Thrinax. Se designaron los lectotipos de Hemithrinax compacta y Leucothrinax morrisii, y se precisaron los lectotipos de otros nueve nombres. Sociedad Cubana de Botánica Calle Cuba 406 e/ Amargura y Brasil, Palabras clave: Arecaceae, Hemithrinax, Leucothrinax, Thrinax La Habana Vieja, La Habana, Cuba A S RAC Recibido: 01/06/2018 Aceptado: 21/01/2019 The list of taxa and nomenclatural synonyms of the genera Hemithrinax, Leucothrinax and Thrinax is updated. The lectotype of Hemithrinax compacta were designated and lectotypes of other ten names were specified. Key words: Arecaceae, Hemithrinax, Leucothrinax, Thrinax INTRODUCCIÓN Hemithrinax es un género endémico cubano, representa- do por tres especies y una variedad reconocida. Sus La familia Arecaceae Schultz Sch. (nom. cons.) está táxones se diferencian fácilmente de los de Thrinax o representada en Cuba por 15 géneros con 80 especies, Leucothrinax por presentar las venas transversales poco ocho híbridos y nueve táxones infraespecíficos (Moya y visibles, mientras que en éstos las venas transversales Leiva, 2000). De ellos, tres constituyen nuevos registros o son conspicuas (Lewis y Zona, 2008). cambios de estatus posteriores (Suárez, 2015; Verdecia, 2016; Moya et al., 2017; Moya y Méndez, 2018), lo cual Leucothrinax es un género monotípico, de distribución sugiere que la riqueza taxonómica de la familia en Cuba caribeña, representado por Leucothrinax morrisii aún no es totalmente conocida.