Great Basin Native Plant Project
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Socioeconomic Monitoring of the Olympic National Forest and Three Local Communities
NORTHWEST FOREST PLAN THE FIRST 10 YEARS (1994–2003) Socioeconomic Monitoring of the Olympic National Forest and Three Local Communities Lita P. Buttolph, William Kay, Susan Charnley, Cassandra Moseley, and Ellen M. Donoghue General Technical Report United States Forest Pacific Northwest PNW-GTR-679 Department of Service Research Station July 2006 Agriculture The Forest Service of the U.S. Department of Agriculture is dedicated to the principle of multiple use management of the Nation’s forest resources for sustained yields of wood, water, forage, wildlife, and recreation. Through forestry research, cooperation with the States and private forest owners, and management of the National Forests and National Grasslands, it strives—as directed by Congress—to provide increasingly greater service to a growing Nation. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all pro- grams.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, 1400 Independence Avenue, SW, Washington, DC 20250-9410 or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer. -

Snowmobiles in the Wilderness
Snowmobiles in the Wilderness: You can help W a s h i n g t o n S t a t e P a r k s A necessary prohibition Join us in safeguarding winter recreation: Each year, more and more people are riding snowmobiles • When riding in a new area, obtain a map. into designated Wilderness areas, which is a concern for • Familiarize yourself with Wilderness land managers, the public and many snowmobile groups. boundaries, and don’t cross them. This may be happening for a variety of reasons: many • Carry the message to clubs, groups and friends. snowmobilers may not know where the Wilderness boundaries are or may not realize the area is closed. For more information about snowmobiling opportunities or Wilderness areas, please contact: Wilderness…a special place Washington State Parks and Recreation Commission (360) 902-8500 Established by Congress through the Wilderness Washington State Snowmobile Association (800) 784-9772 Act of 1964, “Wilderness” is a special land designation North Cascades National Park (360) 854-7245 within national forests and certain other federal lands. Colville National Forest (509) 684-7000 These areas were designated so that an untouched Gifford Pinchot National Forest (360) 891-5000 area of our wild lands could be maintained in a natural Mt. Baker-Snoqualmie National Forest (425) 783-6000 state. Also, they were set aside as places where people Mt. Rainier National Park (877) 270-7155 could get away from the sights and sounds of modern Okanogan-Wenatchee National Forest (509) 664-9200 civilization and where elements of our cultural history Olympic National Forest (360) 956-2402 could be preserved. -

Land Areas of the National Forest System, As of September 30, 2019
United States Department of Agriculture Land Areas of the National Forest System As of September 30, 2019 Forest Service WO Lands FS-383 November 2019 Metric Equivalents When you know: Multiply by: To fnd: Inches (in) 2.54 Centimeters Feet (ft) 0.305 Meters Miles (mi) 1.609 Kilometers Acres (ac) 0.405 Hectares Square feet (ft2) 0.0929 Square meters Yards (yd) 0.914 Meters Square miles (mi2) 2.59 Square kilometers Pounds (lb) 0.454 Kilograms United States Department of Agriculture Forest Service Land Areas of the WO, Lands National Forest FS-383 System November 2019 As of September 30, 2019 Published by: USDA Forest Service 1400 Independence Ave., SW Washington, DC 20250-0003 Website: https://www.fs.fed.us/land/staff/lar-index.shtml Cover Photo: Mt. Hood, Mt. Hood National Forest, Oregon Courtesy of: Susan Ruzicka USDA Forest Service WO Lands and Realty Management Statistics are current as of: 10/17/2019 The National Forest System (NFS) is comprised of: 154 National Forests 58 Purchase Units 20 National Grasslands 7 Land Utilization Projects 17 Research and Experimental Areas 28 Other Areas NFS lands are found in 43 States as well as Puerto Rico and the Virgin Islands. TOTAL NFS ACRES = 192,994,068 NFS lands are organized into: 9 Forest Service Regions 112 Administrative Forest or Forest-level units 503 Ranger District or District-level units The Forest Service administers 149 Wild and Scenic Rivers in 23 States and 456 National Wilderness Areas in 39 States. The Forest Service also administers several other types of nationally designated -

Skagit River Emergency Bank Stabilization And
United States Department of the Interior FISH AND WILDLIFE SERVICE Washington Fish and Wildlife Office 510 Desmond Dr. SE, Suite 102 Lacey, Washington 98503 In Reply Refer To: AUG 1 6 2011 13410-2011-F-0141 13410-2010-F-0175 Daniel M. Mathis, Division Adnlinistrator Federal Highway Administration ATIN: Jeff Horton Evergreen Plaza Building 711 Capitol Way South, Suite 501 Olympia, Washington 98501-1284 Michelle Walker, Chief, Regulatory Branch Seattle District, Corps of Engineers ATIN: Rebecca McAndrew P.O. Box 3755 Seattle, Washington 98124-3755 Dear Mr. Mathis and Ms. Walker: This document transmits the U.S. Fish and Wildlife Service's Biological Opinion (Opinion) based on our review of the proposed State Route 20, Skagit River Emergency Bank Stabilization and Chronic Environmental Deficiency Project in Skagit County, Washington, and its effects on the bull trout (Salve linus conjluentus), marbled murrelet (Brachyramphus marmoratus), northern spotted owl (Strix occidentalis caurina), and designated bull trout critical habitat, in accordance with section 7 of the Endangered Species Act of 1973, as amended (16 U.S.C. 1531 et seq.) (Act). Your requests for initiation of formal consultation were received on February 3, 2011, and January 19,2010. The Federal Highway Administration (FHWA) and U.S. Army Corps of Engineers - Seattle District (Corps) provided information in support of "may affect, likely to adversely affect" determinations for the bull trout and designated bull trout critical habitat. On April 13 and 14, 2011, the FHWA and Corps gave their consent for addressing potential adverse effects to the bull trout and bull trout critical habitat with a single Opinion. -

U·M·I University Microfilms International a 8Ell & Howell Information Company 300 North Zeeb Road
Patterns of homoplasy in North American Astragalus L. (Fabaceae). Item Type text; Dissertation-Reproduction (electronic) Authors Sanderson, Michael John. Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 10/10/2021 18:39:52 Link to Item http://hdl.handle.net/10150/184764 INFORMATION TO USERS The most advanced technology has been used to photo graph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UIVn a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are re produced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. -

December 2012 Number 1
Calochortiana December 2012 Number 1 December 2012 Number 1 CONTENTS Proceedings of the Fifth South- western Rare and Endangered Plant Conference Calochortiana, a new publication of the Utah Native Plant Society . 3 The Fifth Southwestern Rare and En- dangered Plant Conference, Salt Lake City, Utah, March 2009 . 3 Abstracts of presentations and posters not submitted for the proceedings . 4 Southwestern cienegas: Rare habitats for endangered wetland plants. Robert Sivinski . 17 A new look at ranking plant rarity for conservation purposes, with an em- phasis on the flora of the American Southwest. John R. Spence . 25 The contribution of Cedar Breaks Na- tional Monument to the conservation of vascular plant diversity in Utah. Walter Fertig and Douglas N. Rey- nolds . 35 Studying the seed bank dynamics of rare plants. Susan Meyer . 46 East meets west: Rare desert Alliums in Arizona. John L. Anderson . 56 Calochortus nuttallii (Sego lily), Spatial patterns of endemic plant spe- state flower of Utah. By Kaye cies of the Colorado Plateau. Crystal Thorne. Krause . 63 Continued on page 2 Copyright 2012 Utah Native Plant Society. All Rights Reserved. Utah Native Plant Society Utah Native Plant Society, PO Box 520041, Salt Lake Copyright 2012 Utah Native Plant Society. All Rights City, Utah, 84152-0041. www.unps.org Reserved. Calochortiana is a publication of the Utah Native Plant Society, a 501(c)(3) not-for-profit organi- Editor: Walter Fertig ([email protected]), zation dedicated to conserving and promoting steward- Editorial Committee: Walter Fertig, Mindy Wheeler, ship of our native plants. Leila Shultz, and Susan Meyer CONTENTS, continued Biogeography of rare plants of the Ash Meadows National Wildlife Refuge, Nevada. -

Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1
Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1 Authors: Jiang, Wei, He, Hua-Jie, Lu, Lu, Burgess, Kevin S., Wang, Hong, et. al. Source: Annals of the Missouri Botanical Garden, 104(2) : 171-229 Published By: Missouri Botanical Garden Press URL: https://doi.org/10.3417/2019337 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/journals/Annals-of-the-Missouri-Botanical-Garden on 01 Apr 2020 Terms of Use: https://bioone.org/terms-of-use Access provided by Kunming Institute of Botany, CAS Volume 104 Annals Number 2 of the R 2019 Missouri Botanical Garden EVOLUTION OF ANGIOSPERM Wei Jiang,2,3,7 Hua-Jie He,4,7 Lu Lu,2,5 POLLEN. 7. NITROGEN-FIXING Kevin S. Burgess,6 Hong Wang,2* and 2,4 CLADE1 De-Zhu Li * ABSTRACT Nitrogen-fixing symbiosis in root nodules is known in only 10 families, which are distributed among a clade of four orders and delimited as the nitrogen-fixing clade. -

Washington Plant List Douglas County by Scientific Name
The NatureMapping Program Washington Plant List Revised: 9/15/2011 Douglas County by Scientific Name (1) Non- native, (2) ID Scientific Name Common Name Plant Family Invasive √ 763 Acer glabrum Douglas maple Aceraceae 800 Alisma graminium Narrowleaf waterplantain Alismataceae 19 Alisma plantago-aquatica American waterplantain Alismataceae 1087 Rhus glabra Sumac Anacardiaceae 650 Rhus radicans Poison ivy Anacardiaceae 29 Angelica arguta Sharp-tooth angelica Apiaceae 809 Angelica canbyi Canby's angelica Apiaceae 915 Cymopteris terebinthinus Turpentine spring-parsley Apiaceae 167 Heracleum lanatum Cow parsnip Apiaceae 991 Ligusticum grayi Gray's lovage Apiaceae 709 Lomatium ambiguum Swale desert-parsley Apiaceae 997 Lomatium canbyi Canby's desert-parsley Apiaceae 573 Lomatium dissectum Fern-leaf biscuit-root Apiaceae 582 Lomatium geyeri Geyer's desert-parsley Apiaceae 586 Lomatium gormanii Gorman's desert-parsley Apiaceae 998 Lomatium grayi Gray's desert-parsley Apiaceae 999 Lomatium hambleniae Hamblen's desert-parsley Apiaceae 609 Lomatium macrocarpum Large-fruited lomatium Apiaceae 1000 Lomatium nudicaule Pestle parsnip Apiaceae 634 Lomatium triternatum Nine-leaf lomatium Apiaceae 474 Osmorhiza chilensis Sweet-cicely Apiaceae 264 Osmorhiza occidentalis Western sweet-cicely Apiaceae 1044 Osmorhiza purpurea Purple sweet-cicely Apiaceae 492 Sanicula graveolens Northern Sierra) sanicle Apiaceae 699 Apocynum androsaemifolium Spreading dogbane Apocynaceae 813 Apocynum cannabinum Hemp dogbane Apocynaceae 681 Asclepias speciosa Showy milkweed Asclepiadaceae -
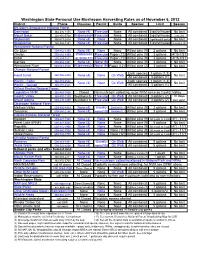
Washington State Personal Use Mushroom Harvesting Rules As of November 6, 2012 District Phone Closures Permit Guide Species Limit Season Mt
Washington State Personal Use Mushroom Harvesting Rules as of November 6, 2012 District Phone Closures Permit Guide Species Limit Season Mt. Baker-Snoqualmie National Forest Darrington 360-436-1155 None (4) Free use None All combined 5 gal/yr/house No limit Mount Baker 360-856-5700 Wilderness(4) Free use None All combined 5 gal/yr/house 2 weeks Skykomish 360-677-2414 None (4) None None All combined 1 gal/day&5 gal/yr No limit Snoqualmie 425-888-1421 None (4) Free use None All combined 5 gal/yr/house 2 weeks (8) Wenatchee National Forest Cle Elum 509-852-1100 None (4) None None All but pine (9) 2 gallons No limit Chelan 509-682-4900 Wilderness Free use Paper (12) All but pine (9) 3 gallons 4/15-7/31 Entiat 509-784-1511 &LSR(4,11) Free use Paper (12) All but pine (9) 3 gallons 4/15-7/31 Naches 509-653-1401 None (4) None (9) None All but pine (9) 3 gallons No limit Wenatchee River 509-548-2550 Wilderness(4) None (9) Paper (12) All but pine (2) 3 gallons No limit Olympic National Forest Each species 1 gallon (1,3) Hood Canal 360-765-2200 None (4) None On Web No limit All combined 3 gallons (1) Pacific - Forks 360-374-6522 Each species 1 gallon (1,3) None (4) None On Web No limit Pacific - Quinalt 360-288-2525 All combined 3 gallons (1) Gifford Pinchot National Forest Legislative NVM 360-449-7800 Closed No mushroom collecting, outer NVM same as Cowlitz Valley Cowlitz Valley 360-497-1100 SeeMap(4,5) Free use On Web All combined 3 gallons (2) 10 days Mount Adams 509-395-3400 SeeMap(4,5) Free use On Web All combined 3 gallons (2) per year Okanogan -

Mckayla Stevens, Donald H. Mansfield James F. Smith Mary Ann E. Feist
RESOLVING THE ANOMALY OF LOMATIUM ANOMALUM: DISCOVERY OF A NEW SPECIES IN SOUTHWESTERN IDAHO (U.S.A.), LOMATIUM ANDRUSIANUM (APIACEAE) Mckayla Stevens, Donald H. Mansfield James F. Smith The College of Idaho Boise State University 2112 Cleveland Blvd. 1910 W. University Drive Caldwell, Idaho 83605, U.S.A. Boise, Idaho 83725, U.S.A. Mary Ann E. Feist University of Wisconsin-Madison 430 Lincoln Drive Madison, Wisconsin 53706, U.S.A. ABSTRACT Apparent polyphyly within the unresolved clade of Lomatium (Apiaceae) containing L. triternatum, L. anomalum, L. thompsonii, and L. pack- ardiae suggests conflict among current taxonomic classification schemes. To recover this clade and more clearly define species boundaries, we examined populations of L. anomalum from three geographic regions in Idaho and adjacent Oregon. Using phylogenetic, morphological, and ecological data, we conclude that the L. anomalum complex currently circumscribes multiple species. Phylogenetic analysis of the nuclear ribosomal ITS and ETS, and cpDNA rpl32-trnLUAG, rps-16 intron, trnD-trnT, ndhA intron, and psbA-trnH recovered populations from the Boise foothills as a distinct, monophyletic clade. Principal Components Analysis of 30 reproductive and vegetative characters show two distinct groups: one of Boise foothills and one of the combined Mann Creek and Camas Prairie vicinities. Principal Components Analysis of 16 soil characteristics show that soils occupied by Boise foothills populations are distinct from those occupied by Mann Creek and Camas Prairie populations. Based on phylogenetic, morphometric, and ecologic criteria, populations of what had been considered L. anomalum from the Boise foothills and vicinity are here described as a new species—Lomatium andrusianum. -

Astragalus Lentiginosus Var. Coachellae
Evaluating the Biological Conservation Status of the Coachella Valley Milkvetch (Astragalus lentiginosus var. coachellae) Robert J. Meinke, Kelly Amsberry, Rebecca E. Currin, Stephen C. Meyers, and Brian Knaus Native Plant Conservation Program OREGON DEPARTMENT OF AGRICULTURE Oregon Department of Agriculture NATIVE PLANT CONSERVATION PROGRAM NATIVE PLANT CONSERVATION PROGRAM and Department of Botany & Plant Pathology Oregon State University, Corvallis October, 2007 EXECUTIVE SUMMARY The Coachella Valley milkvetch (Astragalus lentiginosus var. coachellae) is a federally-listed endangered taxon, restricted to a highly active sand dune environment near the eastern base of the San Jacinto Mountains in the western Sonoran Desert. A recent assessment confirms that var. coachellae is endemic to the Coachella Valley, and that reported populations from the Desert Center area (disjunct ca. 75 km to the east) actually represent A. lentiginosus var. variabilis. Much of the range of A. lentiginosus var. coachellae overlaps the cities of Palm Springs, Cathedral City, and other nearby communities, where remaining populations are threatened by the effects of urbanization and habitat fragmentation. A Habitat Conservation Plan (HCP; in preparation) is expected to place several of the more significant remaining sites within administratively protected reserve areas located outside the urban core. However, little information has been available that describes the natural history and ecological status of Coachella Valley milkvetch populations, and specific management plans have yet to be developed. The current study (conducted in 2005 and 2006) corroborates earlier reports that var. coachellae exhibits a labile life history correlated with annual precipitation, in which short-lived perennials loosely dominate the less arid, northern end of the range and annuals are more prevalent in the drier south. -

Floral Guilds of Bees in Sagebrush Steppe: Comparing Bee Usage Of
ABSTRACT: Healthy plant communities of the American sagebrush steppe consist of mostly wind-polli- • nated shrubs and grasses interspersed with a diverse mix of mostly spring-blooming, herbaceous perennial wildflowers. Native, nonsocial bees are their common floral visitors, but their floral associations and abundances are poorly known. Extrapolating from the few available pollination studies, bees are the primary pollinators needed for seed production. Bees, therefore, will underpin the success of ambitious seeding efforts to restore native forbs to impoverished sagebrush steppe communities following vast Floral Guilds of wildfires. This study quantitatively characterized the floral guilds of 17 prevalent wildflower species of the Great Basin that are, or could be, available for restoration seed mixes. More than 3800 bees repre- senting >170 species were sampled from >35,000 plants. Species of Osmia, Andrena, Bombus, Eucera, Bees in Sagebrush Halictus, and Lasioglossum bees prevailed. The most thoroughly collected floral guilds, at Balsamorhiza sagittata and Astragalus filipes, comprised 76 and 85 native bee species, respectively. Pollen-specialists Steppe: Comparing dominated guilds at Lomatium dissectum, Penstemon speciosus, and several congenerics. In contrast, the two native wildflowers used most often in sagebrush steppe seeding mixes—Achillea millefolium and Linum lewisii—attracted the fewest bees, most of them unimportant in the other floral guilds. Suc- Bee Usage of cessfully seeding more of the other wildflowers studied here would greatly improve degraded sagebrush Wildflowers steppe for its diverse native bee communities. Index terms: Apoidea, Asteraceae, Great Basin, oligolecty, restoration Available for Postfire INTRODUCTION twice a decade (Whisenant 1990). Massive Restoration wildfires are burning record acreages of the The American sagebrush steppe grows American West; two fires in 2007 together across the basins and foothills over much burned >500,000 ha of shrub-steppe and 1,3 James H.