ED 023 604 SE 005 420 by -Liston, Robert H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
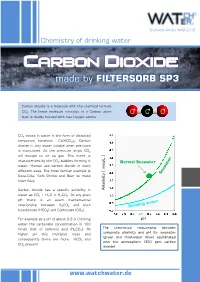
Carbon Dioxide - Made by FILTERSORB SP3
Technical Article MAR 2015 Chemistry of drinking water Carbon dioxide - made by FILTERSORB SP3 Carbon dioxide is a molecule with the chemical formula CO2. The linear molecule consists of a Carbon atom O C O that is doubly bonded with two Oxygen atoms. CO2 exists in water in the form of dissolved temporary hardness Ca(HCO3)2. Carbon dioxide is only water soluble when pressure is maintained. As the pressure drops CO2 will escape to air as gas. This event is characterized by the CO2 bubbles forming in /L ] Normal Seawater water. Human use carbon dioxide in many meq different ways. The most familiar example is Coca-Cola, Soft Drinks and Beer to make them fizzy. Carbon dioxide has a specific solubility in [ Alkalinity water as CO2 + H2O ⇌ H2CO3. At any given pH there is an exact mathematical relationship between H2CO3 and both bicarbonate (HCO3) and Carbonate (CO3). For example at a pH of about 9.3 in Drinking pH water the carbonate concentration is 100 The theoretical relationship between times that of carbonic acid (H2CO3). At carbonate alkalinity and pH for seawater higher pH this multiplier rises and (green and freshwater (blue) equilibrated consequently there are more HCO and 3 with the atmosphere (350 ppm carbon CO present 3 dioxide) www.watchwater.de Water ® Technology FILTERSORB SP3 WATCH WATER & Chemicals Making the healthiest water How SP3 Water functions in human body ? Answer: Carbon dioxide is a waste product of the around these hollow spaces will spasm and respiratory system, and of several other constrict. chemical reactions in the body such as the Transportation of oxygen to the tissues: Oxygen creation of ATP. -

A Complete Guide to Benzene
A Complete Guide to Benzene Benzene is an important organic chemical compound with the chemical formula C6H6. The benzene molecule is composed of six carbon atoms joined in a ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Due to the cyclic continuous pi bond between the carbon atoms, benzene is classed as an aromatic hydrocarbon, the second [n]- annulene ([6]-annulene). It is sometimes abbreviated Ph–H. Benzene is a colourless and highly flammable liquid with a sweet smell. Source: Wikipedia Protecting people and the environment Protecting both people and the environment whilst meeting the operational needs of your business is very important and, if you have operations in the UK you will be well aware of the requirements of the CoSHH Regulations1 and likewise the Code of Federal Regulations (CFR) in the US2. Similar legislation exists worldwide, the common theme being an onus on hazard identification, risk assessment and the provision of appropriate control measures (bearing in mind the hierarchy of controls) as well as health surveillance in most cases. And whilst toxic gasses such as hydrogen sulphide and carbon monoxide are a major concern because they pose an immediate (acute) danger to life, long term exposure to relatively low level concentrations of other gasses or vapours such as volatile organic compounds (VOC) are of equal importance because of the chronic illnesses that can result from that ongoing exposure. Benzene, a common VOC Organic means the chemistry of carbon based compounds, which are substances that results from a combination of two or more different chemical elements. -

Organocatalytic Asymmetric N-Sulfonyl Amide C-N Bond Activation to Access Axially Chiral Biaryl Amino Acids
ARTICLE https://doi.org/10.1038/s41467-020-14799-8 OPEN Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids Guanjie Wang1, Qianqian Shi2, Wanyao Hu1, Tao Chen1, Yingying Guo1, Zhouli Hu1, Minghua Gong1, ✉ ✉ ✉ Jingcheng Guo1, Donghui Wei 2 , Zhenqian Fu 1,3 & Wei Huang1,3 1234567890():,; Amides are among the most fundamental functional groups and essential structural units, widely used in chemistry, biochemistry and material science. Amide synthesis and trans- formations is a topic of continuous interest in organic chemistry. However, direct catalytic asymmetric activation of amide C-N bonds still remains a long-standing challenge due to high stability of amide linkages. Herein, we describe an organocatalytic asymmetric amide C-N bonds cleavage of N-sulfonyl biaryl lactams under mild conditions, developing a general and practical method for atroposelective construction of axially chiral biaryl amino acids. A structurally diverse set of axially chiral biaryl amino acids are obtained in high yields with excellent enantioselectivities. Moreover, a variety of axially chiral unsymmetrical biaryl organocatalysts are efficiently constructed from the resulting axially chiral biaryl amino acids by our present strategy, and show competitive outcomes in asymmetric reactions. 1 Key Laboratory of Flexible Electronics & Institute of Advanced Materials, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, China. 2 College -

Chemical Formula
Chemical Formula Jean Brainard, Ph.D. Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) AUTHOR Jean Brainard, Ph.D. To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Introduction, Formulas and Spectroscopy Overview Problem 1
Chapter 1 – Introduction, Formulas and Spectroscopy Overview Problem 1 (p 12) - Helpful equations: c = ()() and = (1 / ) so c = () / ( ) c = 3.00 x 108 m/sec = 3.00 x 1010 cm/sec a. Which photon of electromagnetic radiation below would have the longer wavelength? Convert both values to meters. -1 = 3500 cm = 4 x 1014 Hz = (1/) = (c / ) = (1/ ) = (3.0 x 108 m/s) / (4 x 1014 s-1) = (1/ cm-1)(1 m/100 cm) = 2.9 x 10-6 m = 7.5 x 10-7 m = longer wavelength = lower energy b. Which photon of electromagnetic radiation below would have the higher frequency? Convert both values to Hz. = 400 cm-1 = 300 nm = (1/ ) = 1/ [(400 cm-1)(100 cm / 1 m)] = (c/) 8 -5 9 = 2.5 x 10-5 m = (3.0 x 10 m/s) / (2.5 x 10 nm)(1m / 10 nm) v = (3.0 x 108 m/s) / (300 nm)(1m / 109 nm) = (c/) v = 1.0 x x 1015 s-1 = (3.0 x 108 m/s) / (2.5 x 10-5 m) v = 1.2 x 1013 s-1 higher frequency c. Which photon of electromagnetic radiation below would have the smaller wavenumber? Convert both values to cm-1. = 1 m = 6 x 1010 Hz = (1 / ) -1 = ( / c) = (1 / 1m) = 1 m-1 100 cm = 100 cm-1 100 cm-1 -1 = (6 x 1010 s-1 / 3.0 x 108 m/s) = 200 m-1 -1 1 m -1 = 20,000 cm smaller wavenumber 1 m Problem 2 (p 14) - Order the following photons from lowest to highest energy (first convert each value to kjoules/mole). -

Reliable Determination of Amidicity in Acyclic Amides and Lactams Stephen A
Article pubs.acs.org/joc Reliable Determination of Amidicity in Acyclic Amides and Lactams Stephen A. Glover* and Adam A. Rosser Department of Chemistry, School of Science and Technology, University of New England, Armidale, NSW 2351, Australia *S Supporting Information ABSTRACT: Two independent computational methods have been used for determination of amide resonance stabilization and amidicities relative to N,N-dimethylacetamide for a wide range of acyclic and cyclic amides. The first method utilizes carbonyl substitution nitrogen atom replacement (COSNAR). The second, new approach involves determination of the difference in amide resonance between N,N-dimethylacetamide and the target amide using an isodesmic trans-amidation process and is calibrated relative to 1-aza-2-adamantanone with zero amidicity and N,N-dimethylace- tamide with 100% amidicity. Results indicate excellent coherence between the methods, which must be regarded as more reliable than a recently reported approach to amidicities based upon enthalpies of hydrogenation. Data for acyclic planar and twisted amides are predictable on the basis of the degrees of pyramidalization at nitrogen and twisting about the C−N bonds. Monocyclic lactams are predicted to have amidicities at least as high as N,N-dimethylacetamide, and the β-lactam system is planar with greater amide resonance than that of N,N- dimethylacetamide. Bicyclic penam/em and cepham/em scaffolds lose some amidicity in line with the degree of strain-induced pyramidalization at the bridgehead nitrogen and twist about the amide bond, but the most puckered penem system still retains substantial amidicity equivalent to 73% that of N,N-dimethylacetamide. ■ INTRODUCTION completed by a third resonance form, III, with no C−N pi Amide linkages are ubiquitous in proteins, peptides, and natural character and on account of positive polarity at carbon and the or synthetic molecules and in most of these they possess, electronegativity of nitrogen, must be destabilizing. -

H2CS) and Its Thiohydroxycarbene Isomer (HCSH
A chemical dynamics study on the gas phase formation of thioformaldehyde (H2CS) and its thiohydroxycarbene isomer (HCSH) Srinivas Doddipatlaa, Chao Hea, Ralf I. Kaisera,1, Yuheng Luoa, Rui Suna,1, Galiya R. Galimovab, Alexander M. Mebelb,1, and Tom J. Millarc,1 aDepartment of Chemistry, University of Hawai’iatManoa, Honolulu, HI 96822; bDepartment of Chemistry and Biochemistry, Florida International University, Miami, FL 33199; and cSchool of Mathematics and Physics, Queen’s University Belfast, Belfast BT7 1NN, Northern Ireland, United Kingdom Edited by Stephen J. Benkovic, The Pennsylvania State University, University Park, PA, and approved August 4, 2020 (received for review March 13, 2020) Complex organosulfur molecules are ubiquitous in interstellar molecular sulfur dioxide (SO2) (21) and sulfur (S8) (22). The second phase clouds, but their fundamental formation mechanisms have remained commences with the formation of the central protostars. Tempera- largely elusive. These processes are of critical importance in initiating a tures increase up to 300 K, and sublimation of the (sulfur-bearing) series of elementary chemical reactions, leading eventually to organo- molecules from the grains takes over (20). The subsequent gas-phase sulfur molecules—among them potential precursors to iron-sulfide chemistry exploits complex reaction networks of ion–molecule and grains and to astrobiologically important molecules, such as the amino neutral–neutral reactions (17) with models postulating that the very acid cysteine. Here, we reveal through laboratory experiments, first sulfur–carbon bonds are formed via reactions involving methyl electronic-structure theory, quasi-classical trajectory studies, and astro- radicals (CH3)andcarbene(CH2) with atomic sulfur (S) leading to chemical modeling that the organosulfur chemistry can be initiated in carbonyl monosulfide and thioformaldehyde, respectively (18). -

Ambient Water Quality Criteria for Polycyclic Aromatic Hydrocarbons (Pahs)
Ambient Water Quality Criteria For Polycyclic Aromatic Hydrocarbons (PAHs) Ministry of Environment, Lands and Parks Province of British Columbia N. K. Nagpal, Ph.D. Water Quality Branch Water Management Division February, 1993 ACKNOWLEDGEMENTS The author is indebted to the following individual and agencies for providing valuable comments during the preparation of this document. Dr. Ray Copes BC. Ministry of Health, Victoria, BC. Dr. G. R. Fox Environmental Protection Div., BC. MOELP, Victoria, BC. Mr. L. W. Pommen Water Quality Branch, BC. MOELP, Victoria, BC. Mr. R. J. Rocchini Water Quality Branch, BC. MOELP, Victoria, BC. Ms. Sherry Smith Eco-Health Branch, Conservation and Protection, Environment Canada, Hull, Quebec Mr. Scott Teed Eco-Health Branch, Conservation and Protection, Environment Canada, Hull, Quebec Ms. Bev Raymond Integrated Programs Branch, Inland Waters, Environment Canada, North Vancouver, BC. 1.0 INTRODUCTION Polycyclic aromatic hydrocarbons (PAHs) are organic compounds which are non- essential for the growth of plants, animals or humans; yet, they are ubiquitous in the environment. When present in sufficient quantity in the environment, certain PAHs are toxic and carcinogenic to plants, animals and humans. This document discusses the characteristics of PAHs and their effects on various water uses, which include drinking Ministry of Environment Water Protection and Sustainability Branch Mailing Address: Telephone: 250 387-9481 Environmental Sustainability PO Box 9362 Facsimile: 250 356-1202 and Strategic Policy Division Stn Prov Govt Website: www.gov.bc.ca/water Victoria BC V8W 9M2 water, aquatic life, wildlife, livestock watering, irrigation, recreation and aesthetics, and industrial water supplies. A significant portion of this document discusses the effects of PAHs upon aquatic life, due to its sensitivity to PAHs. -

The Birth of Modern Chemistry
The Birth of Modern Chemistry 3rd semester project, Fall 2008 NIB Group nr.5, House 13.2 Thomas Allan Rayner & Aiga Mackevica Supervisor: Torben Brauner Abstract Alchemy was a science practiced for more than two millennia up till the end of 18th century when it was replaced by modern chemistry, which is practiced up till this very day. The purpose of this report is to look into this shift and investigate whether this shift can be classified as a paradigm shift according to the famous philosopher Thomas Kuhn, who came up with a theory on the structure of scientific revolutions. In order to come to draw any kind of conclusions, the report summarizes and defines the criteria for what constitutes a paradigm, crisis and paradigm shift, which are all important in order to investigate the manner in which a paradigm shift occurs. The criteria are applied to the historical development of modern chemistry and alchemy as well as the transition between the two sciences. As a result, alchemy and modern chemistry satisfy the requirements and can be viewed as two different paradigms in a Kuhnian sense. However, it is debatable whether the shift from alchemy to modern chemistry can be called a paradigm shift. 2 Acknowledgments We would like to thank Torben Brauner for his guidance throughout the project period as our supervisor. We would also like to thank our opposing group – Paula Melo Paulon Hansen, Stine Hesselholt Sloth and their supervisor Ole Andersen for their advice and critique for our project. 3 Table of Contents 1. Introduction ................................................................................................................................ 5 2. -

The Life and Times of Carbon, Student Workbook
ELL Chemistry Assignment # 2 Name: __________________________________ Period: Teacher:_____________________ Collecting Carbon Lesson 1 | page 1 of 4 Where Can We Find Carbon? Carbon compounds are take up carbon dioxide from three-fourths of the dry weight everywhere around us. They the air, and use photosynthesis of plants is carbohydrates. In are in our foods, plastics, soils, to turn carbon dioxide into other words, when you look at in water, in the air, and in our carbohydrates. Carbohydrates plant matter (the stem, leaves, bodies. Carbon compounds can are carbon compounds that roots, and grains) you are come in three physical forms: store energy in the forms we call looking largely at carbohydrates. gas, liquid, and solid. Plants “starches” and “sugars.” About Animals (including humans) get their carbohydrates by eating plants, but they do not store much of what they consume. In fact, less than 1% of the body weight of animals is made up of carbohydrates. The main forms of carbon are: nonliving (abiotic) in rocks, soils, and sediments, and in water, such as bicarbonate, carbonate; living (biotic), such as plant and animal matter and dead organic matter; and carbon-based gases, such as carbon dioxide (CO2), methane (CH4), and carbon monoxide (CO). Chemists use a special system to describe compounds. They use a chemical formula of symbols that indicate the elements that make up chemical compounds. A chemical formula is also called a “molecular formula.” The chemical formula of carbon dioxide is CO2 indicating one carbon atom bonded to two oxygen atoms. The symbol for carbon is C; for hydrogen is H; for oxygen is O; for silica is Si. -

General Chemistry Course Description
Chemistry 110: General Chemistry Note: this is a representative syllabus for Chemistry 110. It is here for informational purposes. It is not intended to substitute for or replace the syllabus your instructor provides. Course Description: Introduction to the general principles of chemistry for students planning a professional career in chemistry, a related science, the health professions, or engineering. Stoichiometry, atomic structure, chemical bonding and geometry, thermochemistry, gases, types of chemical reactions, statistics. Weekly laboratory exercises emphasize quantitative techniques and complement the lecture material. Weekly discussion sessions focus on homework assignments and lecture material. Prerequisite: Mathematics 055 or equivalent. Previous high school chemistry not required. Taught: Annually, 5 semester credits. Course Components: Lecture, Recitation section, Laboratory Textbook: Steven S. Zumdahl and Susan A. Zumdahl, Chemistry, 5th Ed., Houghton Mifflin Company (New York, 2000). The text is available in the college bookstore. Lab Manual: Chemistry Department Faculty, Chemistry 110 Lab Manual, Fall, 2001 Edition, Kinkos. These may be purchased from Linda Noble, the Chemistry Secretary. Safety Goggles: These must be worn at all times in the laboratory. They can be purchased in the college bookstore. Calculator: Must have logs and exponential capability. Lab Notebook: This is a bound duplicate notebook with numbered pages. It can be purchased in the college bookstore Lecture Schedule 1.1-1.5 introduction, laboratory precision -

Course Syllabus
Course Syllabus Department: Science & Technology Date: January 2015 I. Course Prefix and Number: CHM 205 Course Name: Organic Chemistry I – Lecture Only Credit Hours and Contact Hours: 4 credit hours and 4 (3:0:1) contact hours Catalog Description including pre- and co-requisites: A systematic study of the chemistry of carbon compounds emphasizing reactions, mechanisms, and synthesis with a focus on functional groups, addition reactions to alkenes and alkynes, alcohols and ethers, stereochemistry, nomenclature, acid-base chemistry, reaction kinetics and thermodynamics. Completion of General Chemistry II or equivalent with a grade of C or better is prerequisite. II. Course Outcomes and Objectives Student Learning Outcomes: Upon completion of this course, the student will be able to: Demonstrate an understanding of basic principles of organic chemistry and how they relate to everyday experiences. Demonstrate problem solving and critical thinking skills Apply methods of scientific inquiry. Apply problem solving techniques to real-world problems. Demonstrate an understanding of the chemical environment and the role that organic molecules play in the natural and the synthetic world. Relationship to Academic Programs and Curriculum: This course is required for majors in chemistry, chemical engineering, biology, biotechnology, pharmacology, and other pre-professional programs. College Learning Outcomes Addressed by the Course: writing computer literacy oral communications ethics/values X reading citizenship mathematics global concerns X critical thinking information resources 1 III. Instructional Materials and Methods Types of Course Materials: A standard two-semester 200 level organic textbook and workbook. Methods of Instruction (e.g. Lecture and Seminar …): Three hours of lecture, with a one hour recitation period for individual as well as group learning activities such as case studies and guided learning activities.