N-Wasp Regulates Oligodendrocyte Myelination
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Vivo Imaging of Microglia-Mediated Axonal Pruning and Modulation By
Combined bioRxivsingle preprintmanuscript doi: https://doi.org/10.1101/2020.06.07.087221 file ; this version posted June 8, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 In vivo imaging of microglia-mediated axonal pruning and modulation 2 by the complement system 3 Tony K.Y. Lim1 and Edward S. Ruthazer1,2,* 4 1. Department of Neurology & Neurosurgery, Montreal Neurological Institute-Hospital, McGill 5 University, Montreal, Quebec, H3A 2B4; Canada 6 2. Lead Contact 7 *Correspondence: [email protected] 8 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.06.07.087221; this version posted June 8, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 9 Summary 10 Partial phagocytosis – called trogocytosis – of axons by microglia has been documented in ex vivo 11 preparations but has yet to be observed in vivo. Fundamental questions regarding the mechanisms that 12 modulate axon trogocytosis as well as its function in neural circuit development remain unanswered. 13 Here we used 2-photon live imaging of the developing Xenopus laevis retinotectal circuit to observe 14 axon trogocytosis by microglia in vivo. Amphibian regulator of complement activation 3 (aRCA3) was 15 identified as a neuronally expressed, synapse-associated complement inhibitory molecule. 16 Overexpression of aRCA3 enhanced axonal arborization and inhibited trogocytosis, while expression of 17 VAMP2-C3, a complement-enhancing fusion protein tethered to the axon surface, reduced axonal 18 arborization. -

Oligodendrocytes in Development, Myelin Generation and Beyond
cells Review Oligodendrocytes in Development, Myelin Generation and Beyond Sarah Kuhn y, Laura Gritti y, Daniel Crooks and Yvonne Dombrowski * Wellcome-Wolfson Institute for Experimental Medicine, Queen’s University Belfast, Belfast BT9 7BL, UK; [email protected] (S.K.); [email protected] (L.G.); [email protected] (D.C.) * Correspondence: [email protected]; Tel.: +0044-28-9097-6127 These authors contributed equally. y Received: 15 October 2019; Accepted: 7 November 2019; Published: 12 November 2019 Abstract: Oligodendrocytes are the myelinating cells of the central nervous system (CNS) that are generated from oligodendrocyte progenitor cells (OPC). OPC are distributed throughout the CNS and represent a pool of migratory and proliferative adult progenitor cells that can differentiate into oligodendrocytes. The central function of oligodendrocytes is to generate myelin, which is an extended membrane from the cell that wraps tightly around axons. Due to this energy consuming process and the associated high metabolic turnover oligodendrocytes are vulnerable to cytotoxic and excitotoxic factors. Oligodendrocyte pathology is therefore evident in a range of disorders including multiple sclerosis, schizophrenia and Alzheimer’s disease. Deceased oligodendrocytes can be replenished from the adult OPC pool and lost myelin can be regenerated during remyelination, which can prevent axonal degeneration and can restore function. Cell population studies have recently identified novel immunomodulatory functions of oligodendrocytes, the implications of which, e.g., for diseases with primary oligodendrocyte pathology, are not yet clear. Here, we review the journey of oligodendrocytes from the embryonic stage to their role in homeostasis and their fate in disease. We will also discuss the most common models used to study oligodendrocytes and describe newly discovered functions of oligodendrocytes. -

Neuregulin 1–Erbb2 Signaling Is Required for the Establishment of Radial Glia and Their Transformation Into Astrocytes in Cerebral Cortex
Neuregulin 1–erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex Ralf S. Schmid*, Barbara McGrath*, Bridget E. Berechid†, Becky Boyles*, Mark Marchionni‡, Nenad Sˇ estan†, and Eva S. Anton*§ *University of North Carolina Neuroscience Center and Department of Cell and Molecular Physiology, University of North Carolina School of Medicine, Chapel Hill, NC 27599; †Department of Neurobiology, Yale University School of Medicine, New Haven, CT 06510; and ‡CeNes Pharamceuticals, Inc., Norwood, MA 02062 Communicated by Pasko Rakic, Yale University School of Medicine, New Haven, CT, January 27, 2003 (received for review December 12, 2002) Radial glial cells and astrocytes function to support the construction mine whether NRG-1-mediated signaling is involved in radial and maintenance, respectively, of the cerebral cortex. However, the glial cell development and differentiation in the cerebral cortex. mechanisms that determine how radial glial cells are established, We show that NRG-1 signaling, involving erbB2, may act in maintained, and transformed into astrocytes in the cerebral cortex are concert with Notch signaling to exert a critical influence in the not well understood. Here, we show that neuregulin-1 (NRG-1) exerts establishment, maintenance, and appropriate transformation of a critical role in the establishment of radial glial cells. Radial glial cell radial glial cells in cerebral cortex. generation is significantly impaired in NRG mutants, and this defect can be rescued by exogenous NRG-1. Down-regulation of expression Materials and Methods and activity of erbB2, a member of the NRG-1 receptor complex, leads Clonal Analysis to Study NRG’s Role in the Initial Establishment of to the transformation of radial glial cells into astrocytes. -

Microglia Control Glutamatergic Synapses in the Adult Mouse Hippocampus
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.01.429096; this version posted February 2, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Microglia control glutamatergic synapses in the adult mouse hippocampus Short title: Microglia and glutamatergic synapses Bernadette Basilico1†*‡, Laura Ferrucci1‡, Patrizia Ratano2‡, Maria T. Golia1, Alfonso Grimaldi3, Maria Rosito3, Valentina Ferretti4, Ingrid Reverte1,5, Maria C. Marrone6, Maria Giubettini3,7, Valeria De Turris3, Debora Salerno3, Stefano Garofalo1, Marie-Kim St-Pierre8, Micael Carrier8, Massimiliano Renzi1, Francesca Pagani3, Marcello Raspa9, Ferdinando Scavizzi9, Cornelius T. Gross10, Silvia Marinelli5, Marie E. Tremblay8,11, Daniele Caprioli1,5, Laura Maggi1, Cristina Limatola1,2, Silvia Di Angelantonio1,3§, Davide Ragozzino1,5*§ 1Department of Physiology and Pharmacology, Sapienza University of Rome, Rome, Italy. 2IRCCS Neuromed, Via Atinese 18, 86077, Pozzilli, IS, Italy. 3Center for Life Nanoscience, Istituto Italiano di Tecnologia, Rome, Italy. 4Dipartimento di Biologia e Biotecnologie "Charles Darwin", Sapienza University of Rome, Rome, Italy. 5Santa Lucia Foundation (IRCCS Fondazione Santa Lucia), Rome, Italy. 6European Brain Research Institute-Rita Levi Montalcini, Rome, Italy. 7CrestOptics S.p.A., Via di Torre Rossa 66, 00165 Rome, Italy. 8Centre de Recherche du CHU de Québec, Axe Neurosciences Québec, QC, Canada; Département de médecine moléculaire, Université Laval Québec, QC, Canada. 9National Research Council, Institute of Biochemistry and Cell Biology (CNR- IBBC/EMMA/Infrafrontier/IMPC), International Campus “A. Buzzati-Traverso”, Monterotondo (Rome) Italy. -

Pre-Oligodendrocytes from Adult Human CNS
The Journal of Neuroscience, April 1992, 12(4): 1538-l 547 Pre-Oligodendrocytes from Adult Human CNS Regina C. Armstrong,lJ Henry H. Dorn, l,b Conrad V. Kufta,* Emily Friedman,3 and Monique E. Dubois-Dalcq’ ‘Laboratory of Viral and Molecular Pathogenesis, and %urgical Neurology Branch, National Institute of Neurological Disorders and Stroke, Bethesda, Maryland 20892 and 3Department of Neurosurgery, University of Pennsylvania, Philadelphia, Pennsylvania 19104-3246 CNS remyelination and functional recovery often occur after Rapid and efficient neurotransmission is dependent upon the experimental demyelination in adult rodents. This has been electrical insulating capacity of the myelin sheath around axons attributed to the ability of mature oligodendrocytes and/or (reviewed in Ritchie, 1984a,b). Nerve conduction is impaired their precursor cells to divide and regenerate in response after loss of the myelin sheath and results in severe neurological to signals in demyelinating lesions. To determine whether dysfunction in human demyelinating diseases such as multiple oligodendrocyte precursor cells exist in the adult human sclerosis (MS). Remyelination can occur in the CNS of MS CNS, we have cultured white matter from patients under- patients but appears to be limited (Perier and Gregoire, 1965; going partial temporal lobe resection for intractable epilep- Prineas et al., 1984). Studies of acute MS cases have revealed sy. These cultures contained a population of process-bear- that recent demyelinating lesions can exhibit remyelination that ing cells that expressed antigens recognized by the 04 appears to correlate with the generation of new oligodendrocytes monoclonal antibody, but these cells did not express galac- (Prineas et al., 1984; Raine et al., 1988). -

Neuroglial Response to Neuron Injury. a Study Using Intraneural Injection of Ricinus Communis Agglutinin-60
J. Anat. (1989), 164, pp. 201-213 201 With 16 figures Printed in Great Britain Neuroglial response to neuron injury. A study using intraneural injection of ricinus communis agglutinin-60 E. A. LING, C. Y. WEN*, J. Y. SHIEH*, T. Y. YICK AND S. K. LEONG Department of Anatomy, Faculty of Medicine, National University of Singapore, Singapore 0511 and * Department of Anatomy, College of Medicine, National Taiwan University, Taipei, Taiwan 10018 (Accepted 27 September 1988) INTRODUCTION Several studies have shown that ricinus communis agglutinin (RCA), when injected into a nerve in minute amounts, is retrogradely transported by axons in the nerve, resulting in a selective destruction of the parental cell bodies. Thus, the administration of RCA into the vagus nerve would cause a selective destruction of the efferent neurons in the dorsal motor nucleus (Wiley, Blessing & Reis, 1982; Ling & Leong, 1987, 1988). Such a 'suicide transport' of the toxic lectin is also evident in sensory neurons (Yamamoto, Iwasaki & Konno, 1983, 1984; Johnson, Westrum, Henry & Canfield, 1985; Wiley & Oeltmann, 1986). Recently, the use of RCA has become increasingly important as a research tool for tracing the central projections of primary afferents of peripheral nerves (Yamamoto et al. 1983; Leong & Tan, 1987; Ling & Leong, 1987). While much is known about the consequent death of neurons following RCA application, little is known about the response of the non-neuronal cells either closely associated with, or in the vicinity of, the degenerating neurons. According to Yamamoto et al. (1984), the selective destruction of neurons in the trigeminal and dorsal root ganglia by RCA could possibly stimulate the capsule cells involved in the phagocytosis of the degenerating nerve cells. -

University International
INFORMATION TO USERS This was produced from a copy of a document sent to us for microfilming. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help you understand markings or notations which may appear on this reproduction. 1. The sign or “target” for pages apparently lacking from the document photographed is “Missing Page(s)”. If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure you of complete continuity. 2. When an image on the film is obliterated with a round black mark it is an indication that the film inspector noticed either blurred copy because of movement during exposure, or duplicate copy. Unless we meant to delete copyrighted materials that should not have been filmed, you will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., is part of the material being photo graphed the photographer has followed a definite method in “sectioning” the material. It is customary to begin filming at the upper left hand corner of a large sheet and to continue from left to right in equal sections with small overlaps. If necescary, sectioning is continued again—beginning below the first row and continuing on until complete. 4. For any illustrations that cannot be reproduced satisfactorily by xerography, photographic prints can be purchased at additional cost and tipped into your xerographic copy. -

A System for Studying Mechanisms of Neuromuscular Junction Development and Maintenance Valérie Vilmont1,‡, Bruno Cadot1, Gilles Ouanounou2 and Edgar R
© 2016. Published by The Company of Biologists Ltd | Development (2016) 143, 2464-2477 doi:10.1242/dev.130278 TECHNIQUES AND RESOURCES RESEARCH ARTICLE A system for studying mechanisms of neuromuscular junction development and maintenance Valérie Vilmont1,‡, Bruno Cadot1, Gilles Ouanounou2 and Edgar R. Gomes1,3,*,‡ ABSTRACT different animal models and cell lines (Chen et al., 2014; Corti et al., The neuromuscular junction (NMJ), a cellular synapse between a 2012; Lenzi et al., 2015) with the hope of recapitulating some motor neuron and a skeletal muscle fiber, enables the translation of features of neuromuscular diseases and understanding the triggers chemical cues into physical activity. The development of this special of one of their common hallmarks: the disruption of the structure has been subject to numerous investigations, but its neuromuscular junction (NMJ). The NMJ is one of the most complexity renders in vivo studies particularly difficult to perform. studied synapses. It is formed of three key elements: the lower motor In vitro modeling of the neuromuscular junction represents a powerful neuron (the pre-synaptic compartment), the skeletal muscle (the tool to delineate fully the fine tuning of events that lead to subcellular post-synaptic compartment) and the Schwann cell (Sanes and specialization at the pre-synaptic and post-synaptic sites. Here, we Lichtman, 1999). The NMJ is formed in a step-wise manner describe a novel heterologous co-culture in vitro method using rat following a series of cues involving these three cellular components spinal cord explants with dorsal root ganglia and murine primary and its role is basically to ensure the skeletal muscle functionality. -

The Functional Organization of Descending Sensory-Motor 2 Pathways in Drosophila 3 4 5 Shigehiro Namiki,1 Michael H
bioRxiv preprint doi: https://doi.org/10.1101/231696; this version posted December 11, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The functional organization of descending sensory-motor 2 pathways in Drosophila 3 4 5 Shigehiro Namiki,1 Michael H. Dickinson,2 Allan M. Wong,1 Wyatt Korff,1 Gwyneth M. 6 Card,1,* 7 8 1Janelia Research Campus, Howard Hughes Medical Institute, Ashburn, VA 20147, USA 9 2Division of Biology and Bioengineering, California Institute of Technology, Pasadena, CA 91125, USA 10 11 12 This manuscript includes 55 pages of typescript, 15 figures, 0 tables, 24 supplemental figures, and 6 13 supplementary tables. 14 15 KEYWORDS: command neuron; descending neuron; motor control; neuron database; ventral 16 nervous system 17 18 *Correspondence should be addressed to [email protected] (G.M.C) 19 1 bioRxiv preprint doi: https://doi.org/10.1101/231696; this version posted December 11, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 20 SUMMARY 21 22 In most animals, the brain controls the body via a set of descending neurons (DNs) that traverse the neck 23 and terminate in post-cranial regions of the nervous system. This critical neural population is thought to 24 activate, maintain and modulate locomotion and other behaviors. Although individual members of this 25 cell class have been well-studied across species ranging from insects to primates, little is known about the 26 overall connectivity pattern of DNs as a population. -

View Software
TLR4-activated microglia have divergent effects on oligodendrocyte lineage cells DISSERTATION Presented in partial fulfillment of the requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University Evan Zachary Goldstein, BA Neuroscience Graduate Program The Ohio State University 2016 Dissertation Committee: Dr. Dana McTigue, Advisor Dr. Phillip Popovich Dr. Jonathan Godbout Dr. Courtney DeVries Copyright by Evan Zachary Goldstein 2016 i Abstract Myelin accelerates action potential conduction velocity and provides essential metabolic support for axons. Unfortunately, myelin and myelinating cells are often vulnerable to injury or disease, resulting in myelin damage, which in turn can lead to axon dysfunction, overt pathology and neurological impairment. Inflammation is a common component of CNS trauma and disease, and therefore an active inflammatory response is often considered deleterious to myelin health. While inflammation can certainly damage myelin, inflammatory processes also benefit oligodendrocyte (OL) lineage progression and myelin repair. Consistent with the divergent nature of inflammation, intraspinal toll-like receptor 4 (TLR4) activation, an innate immune pathway, kills OL lineage cells, but also initiates oligodendrogenesis. Soluble factors produced by TLR4-activated microglia can reproduce these effects in vitro, however the exact factors are unknown. To determine what microglial factors might contribute to TLR4-induced OL loss and oligodendrogenesis, mRNA of factors known to affect OL lineage cells was quantified in TLR4-activated microglia and spinal cords (chapter 2). Results indicate that TLR4-activated microglia transcribe numerous factors that induce OL loss, OL progenitor cell (OPC) proliferation and OPC differentiation. However, some factors upregulated after intraspinal TLR4 activation were not ii upregulated by microglia, suggesting that other cell types contribute to transcriptional changes in vivo. -
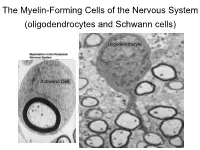
The Myelin-Forming Cells of the Nervous System (Oligodendrocytes and Schwann Cells)
The Myelin-Forming Cells of the Nervous System (oligodendrocytes and Schwann cells) Oligodendrocyte Schwann Cell Oligodendrocyte function Saltatory (jumping) nerve conduction Oligodendroglia PMD PMD Saltatory (jumping) nerve conduction Investigating the Myelinogenic Potential of Individual Oligodendrocytes In Vivo Sparse Labeling of Oligodendrocytes CNPase-GFP Variegated expression under the MBP-enhancer Cerebral Cortex Corpus Callosum Cerebral Cortex Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Ant Commissure Corpus Callosum Cerebral Cortex Caudate Putamen Piriform Cortex Corpus Callosum Ant Commissure Characterization of Oligodendrocyte Morphology Cerebral Cortex Corpus Callosum Caudate Putamen Cerebellum Brain Stem Spinal Cord Oligodendrocytes in disease: Cerebral Palsy ! CP major cause of chronic neurological morbidity and mortality in children ! CP incidence now about 3/1000 live births compared to 1/1000 in 1980 when we started intervening for ELBW ! Of all ELBW {gestation 6 mo, Wt. 0.5kg} , 10-15% develop CP ! Prematurely born children prone to white matter injury {WMI}, principle reason for the increase in incidence of CP ! ! 12 Cerebral Palsy Spectrum of white matter injury ! ! Macro Cystic Micro Cystic Gliotic Khwaja and Volpe 2009 13 Rationale for Repair/Remyelination in Multiple Sclerosis Oligodendrocyte specification oligodendrocytes specified from the pMN after MNs - a ventral source of oligodendrocytes -

Structural and Developmental Principles of Neuropil Assembly in C
Article Structural and developmental principles of neuropil assembly in C. elegans https://doi.org/10.1038/s41586-020-03169-5 Mark W. Moyle1, Kristopher M. Barnes2, Manik Kuchroo3, Alex Gonopolskiy3, Leighton H. Duncan1, Titas Sengupta1, Lin Shao1, Min Guo4, Anthony Santella2, Received: 21 April 2020 Ryan Christensen4, Abhishek Kumar5, Yicong Wu4, Kevin R. Moon6, Guy Wolf7, Accepted: 12 November 2020 Smita Krishnaswamy3,10, Zhirong Bao2,10, Hari Shroff4,5,10, William A. Mohler8,10 & Daniel A. Colón-Ramos1,5,9,10 ✉ Published online: xx xx xxxx Check for updates Neuropil is a fundamental form of tissue organization within the brain1, in which densely packed neurons synaptically interconnect into precise circuit architecture2,3. However, the structural and developmental principles that govern this nanoscale precision remain largely unknown4,5. Here we use an iterative data coarse-graining algorithm termed ‘difusion condensation’6 to identify nested circuit structures within the Caenorhabditis elegans neuropil, which is known as the nerve ring. We show that the nerve ring neuropil is largely organized into four strata that are composed of related behavioural circuits. The stratifed architecture of the neuropil is a geometrical representation of the functional segregation of sensory information and motor outputs, with specifc sensory organs and muscle quadrants mapping onto particular neuropil strata. We identify groups of neurons with unique morphologies that integrate information across strata and that create neural structures that cage the strata within the nerve ring. We use high resolution light-sheet microscopy7,8 coupled with lineage-tracing and cell-tracking algorithms9,10 to resolve the developmental sequence and reveal principles of cell position, migration and outgrowth that guide stratifed neuropil organization.