Reactive Replacement and Addition of Cations in Bioclastic Silica and Calcite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

What Is the Value of Water Contact Angle on Silicon?
materials Article What Is the Value of Water Contact Angle on Silicon? Paweł Bryk 1, Emil Korczeniewski 2, Grzegorz S. Szyma ´nski 2, Piotr Kowalczyk 3, Konrad Terpiłowski 4 and Artur P. Terzyk 2,* 1 Department of Chemistry, Chair of Theoretical Chemistry, Maria Curie-Skłodowska University, 20-031 Lublin, Poland; [email protected] 2 Faculty of Chemistry, Physicochemistry of Carbon Materials Research Group, Nicolaus Copernicus University in Toru´n,Gagarin Street 7, 87-100 Toru´n,Poland; [email protected] (E.K.); [email protected] (G.S.S.) 3 College of Science, Health, Engineering and Education, Murdoch University, Murdoch WA 6150, Australia; [email protected] 4 Department of Chemistry, Chair of Physical Chemistry of Interfacial Phenomena, Maria Curie-Skłodowska University, 20-031 Lublin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-56-61-14-371 Received: 4 March 2020; Accepted: 26 March 2020; Published: 27 March 2020 Abstract: Silicon is a widely applied material and the wetting of silicon surface is an important phenomenon. However, contradictions in the literature appear considering the value of the water contact angle (WCA). The purpose of this study is to present a holistic experimental and theoretical approach to the WCA determination. To do this, we checked the chemical composition of the silicon (1,0,0) surface by using the X-ray photoelectron spectroscopy (XPS) method, and next this surface was purified using different cleaning methods. As it was proved that airborne hydrocarbons change a solid wetting properties the WCA values were measured in hydrocarbons atmosphere. Next, molecular dynamics (MD) simulations were performed to determine the mechanism of wetting in this atmosphere and to propose the force field parameters for silica wetting simulation. -

MAGNESIUM SILICATE, Synthetic
MAGNESIUM SILICATE, synthetic Prepared at the 74th JECFA (2011) and published in FAO Monographs 11 (2011) superseding specifications prepared at the 61st JECFA (2003), published in the Combined Compendium of Food Additive Specifications, FAO JECFA Monographs 1 (2005). An ADI ‘not specified’ was established at the 25th JECFA (1981). SYNONYMS INS No. 553(i) DEFINITION Magnesium silicate (synthetic) is manufactured by the precipitation reaction between sodium silicate and a soluble magnesium salt. The aqueous suspension of the precipitate is filtered and the collected solid washed, dried, classified for particle size and packaged. The finest material is intended for use as an anticaking agent and the coarser particles are for use as a filtering aid. The moisture content of the material meant for use as an anticaking agent is kept to less than 15%. Although magnesium silicate is of variable composition, the molar ratio of MgO to SiO2 is approximately 2:5. Chemical name Magnesium silicate C.A.S. number 1343-88-0 Assay Not less than 15% of MgO and not less than 67% of SiO2, calculated on the ignited basis. DESCRIPTION Very fine, white, odourless powder, free from grittiness FUNCTIONAL USES Anticaking agent, filtering aid CHARACTERISTICS IDENTIFICATION Solubility (Vol. 4) Insoluble in water pH (Vol. 4) 7.0-11.0 (1 in 10 slurry) Magnesium (Vol. 4) Mix about 0.5 g of the sample with 10 ml of dilute hydrochloric acid TS, filter, and neutralize the filtrate to litmus paper with ammonia TS. The neutralized filtrate gives a positive test for magnesium. Silicate Prepare a bead by fusing a few crystals of sodium ammonium phosphate on a platinum loop in the flame of a Bunsen burner. -

Recovery of Silicon from Silica Fume
TSpace Research Repository tspace.library.utoronto.ca Recovery of silicon from silica fume M. Barati, S. Sarder, A. McLean and R. Roy Version Post-print/Accepted Manuscript Citation Barati, M., S. Sarder, A. McLean, and R. Roy. "Recovery of silicon from (published version) silica fume." Journal of Non-Crystalline Solids 357, no. 1 (2011): 18-23. DOI: 10.1016/j.jnoncrysol.2010.09.079 Copyright/License This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. How to cite TSpace items Always cite the published version, so the author(s) will receive recognition through services that track citation counts, e.g. Scopus. If you need to cite the page number of the author manuscript from TSpace because you cannot access the published version, then cite the TSpace version in addition to the published version using the permanent URI (handle) found on the record page. This article was made openly accessible by U of T Faculty. Please tell us how this access benefits you. Your story matters. Recovery of Silicon from Silica Fume M. Barati*, S. Sarder, A. McLean, R. Roy University of Toronto, Dept. of Materials Science and Engineering 140 – 184 College Street, Toronto, ON Canada M5S 3E4 Tel: (416) 978 – 5637 Fax: (416) 978 – 4155 Email: [email protected] Feasibility of producing high purity silicon from amorphous silica fume, using a low temperature magnesiothermic reduction was demonstrated. Commercial silica fume containing 97.5% amorphous silica was first purified by acid leaching and roasting to remove large quantities of transition metals and carbon. -

Making Silicon and Silanes from Sand Description Magnesium and Sand Are Heated Together and Silicon Is Produced by an Exothermic Reaction
Making silicon and silanes from sand Description Magnesium and sand are heated together and silicon is produced by an exothermic reaction. The product is placed in acid to remove magnesium oxide and unreacted magnesium. Small amounts of silanes are produced by the reaction of magnesium silicide (a side product) with the acid. These react spontaneously with air to give spectacular but harmless small explosions. This experiment should take around 5-10 minutes. Apparatus and chemicals • Eye protection • Safety screen • One pyrex test-tube, approximately 150 mm x 17 mm • Clamp and stand • Bunsen burner • One 250 cm3 beaker • One 250 cm3 conical flask • Filter funnel and filter paper. • Access to oven • Desiccator • Access to top pan balance. The quantities given are for one demonstration. • 1 g of dry magnesium powder (Highly flammable) • 1 g of dry silver sand • About 50 cm3 of approximately 2 mol dm–3 hydrochloric acid Technical notes Magnesium powder (Highly flammable). Refer to SSERC or CLEAPSS Hazcards. Dilute hydrochloric acid (Irritant at concentration used). Refer to SSERC or CLEAPSS Recipe and Hazcards . Procedure Health & Safety: Wear safety goggles. Use a safety screen between the apparatus and the audience. Magnesium powder burns vigorously in air. The dust from magnesium powder may be hazardous. Ensure that the mixed powders are absolutely dry before the reaction. It is the responsibility of the teacher to check the employer’s risk assessment. © Royal Society of Chemistry, registered charity number 207890. Before the demonstration a It is important that the reactants are dry. Dry the magnesium powder and the sand for a few hours in an oven at about 100 °C. -
Sio2,Silicon Dioxide Patinal®Us
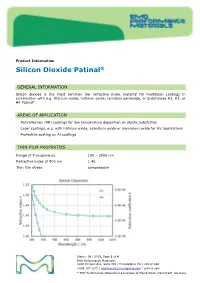
Product Information Silicon Dioxide Patinal ® GENERAL INFORMATION Silicon dioxide is the most common low refractive index material for multilayer coatings in combination with e.g. titanium oxide, hafnium oxide, tantalum pentoxide, or Substances H1, H2, or H4 Patinal . AREAS OF APPLICATION • Antireflection (AR) coatings for low temperature deposition on plastic substrates • Laser coatings, e.g. with hafnium oxide, scandium oxide or aluminium oxide for UV applications • Protective coating on Al-coatings THIN FILM PROPERTIES Range of Transparency 190 – 2000 nm Refractive index at 500 nm 1.46 Thin film stress compressive Status: 08 / 2019, Page 1 of 4 EMD Performance Materials* 1200 Intrepid Ave, Suite 300 / Philadelphia, PA / 19112 USA +888 367 3275 / [email protected] / patinal.com * EMD Performance Materials is a business of Merck KGaA, Darmstadt, Germany wavl / nm 200 250 350 500 650 800 1000 1250 n 1.551 1.531 1.487 1.470 1.463 1.461 1.458 1.457 k 7.1-04 2.6E-04 < 1E-4 < 1E-4 < 1E-4 < 1E-4 < 1E-4 < 1E-4 The resulting optical properties of the thin film are dependent on process conditions like deposition rate and substrate temperature. SiO 2 thin films exhibit an amorphous structure. High packing densities can be achieved by using IAD or a high substrate temperature. NOTES FOR EVAPORATION Evaporator source Electron beam evaporator Crucible / Boat Copper crucible Evaporation temperature 1800 – 2200 °C Deposition rate 0.4 – 1.2 nm/s Oxygen partial pressure none to ~5·10 -5 mbar IAD settings (Leybold APS) 130 – 150 V Bias Substrate temperature conventional RT to 350 °C IAD @ RT QCR-settings Density 2.648 g/cm³, z-ratio 1.0 The evaporation characteristics and the properties of the coating depend on the focusing of the electron beam. -

(Quartz) Silica, Sio2, Is a White Or Colorless Crystalline Compound
Silica (quartz) Silica, SiO2, is a white or colorless crystalline compound found mainly as quartz, sand, flint, and many other minerals. Silica is an important ingredient to manufacture a wide variety of materials. Quartz; Quartz is the most abundant silica mineral. Pure Quartz is colorless and transparent. It occurs in most igneous and practically all metamorphic and sedimentary rocks. It is used as a component of numerous industrial materials. Silicon (Si) has the atomic number 14 and is closely related to carbon. It is a relatively inert metalloid. Silicon is often used for microchips, glass, cement, and pottery. Silica is the most abundant mineral found in the crust of the earth. One of the most common uses of silica quarts is the manufacturer of glass. Silica is the fourteenth element on the periodic table. It can sometimes be found as the substance, quartz which is usually used in jewelry, test tubes, and when placed under pressure, generates an electrical charge. Quartz is the second most abundant mineral in the Earth's crust. It is a clear, glossy mineral with a hardness of 7 on the MOHS scale. Silica, Sa,is a component of glass and concrete. A form of Silica commonly known as quartz, Silica tetrahedra, is the second most common mineral in the earth's crust, it comes in many different forms. Silica is a compound of silicon and oxygen. Earth's outer crust contains 59% of this material. It has three major rock forms, which are quartz, tridymite, and cristobalite. Silica, commonly known in the form of quartz, is the dioxide form of silicon, SiO2. -

Wettability of Silicon, Silicon Dioxide, and Organosilicate Glass
WETTABILITY OF SILICON, SILICON DIOXIDE, AND ORGANOSILICATE GLASS Nelson Martinez, B.S. Thesis Prepared for the Degree of MASTER OF SCIENCE UNIVERSITY OF NORTH TEXAS December 2009 APPROVED: Richard F. Reidy, Major Professor and Interim Chair of the Department of Materials Science and Engineering Witold Brostow, Committee Member Jincheng Du, Committee Member Srinivasan G. Srivilliputhur, Committee Member Costas Tsatsoulis, Dean of the College of Engineering Michael Monticino, Dean of the Robert B. Toulouse School of Graduate Studies Martinez, Nelson. Wettability of Silicon, Silicon Dioxide, and Organosilicate Glass. Master of Science (Materials Science and Engineering), December 2009, 106 pp., 26 tables, 48 illustrations, references 88 titles. Wetting of a substance has been widely investigated since it has many applications to many different fields. Wetting principles can be applied to better select cleans for front end of line (FEOL) and back end of line (BEOL) cleaning processes. These principles can also be used to help determine processes that best repel water from a semiconductor device. It is known that the value of the dielectric constant in an insulator increases when water is absorbed. These contact angle experiments will determine which processes can eliminate water absorption. Wetting is measured by the contact angle between a solid and a liquid. It is known that roughness plays a crucial role on the wetting of a substance. Different surface groups also affect the wetting of a surface. In this work, it was investigated how wetting was affected by different solid surfaces with different chemistries and different roughness. Four different materials were used: silicon; thermally grown silicon dioxide on silicon; chemically vapor deposited (CVD) silicon dioxide on silicon made from tetraethyl orthosilicate (TEOS); and organosilicate glass (OSG) on silicon. -

Silicon Oxide Etch Process
Silicon Oxide Etch Process To remove the areas of silicon dioxide unprotected by photoresist to remove SiO2 and expose the silicon underneath. Preparation and Precaution: 1. This operation should be started within 30 minutes of the completion of the hard bake of the photoresist. 2. Use Buffered Oxide Etch (BOE) solution (6 parts 40% NH4F and 1 part 49% HF). This reagent is found under the last acid fume hood in the Yellow Room. 2.1 Always use plastic beakers and graduated cylinders for etchants containing HF; HF WILL ETCH GLASS! 3. Know the oxide thickness on your substrate. Procedure: 1. Clean the substrate or wafer surface by rinsing with DI water; hold the sample with Teflon tweezers. 2. Before beginning the etching, calculate the time needed to etch through the oxide of known thickness using the known etch rate of the BOE solution; the etch rate at room temperature is ~700 A/min. 3. Use a stop watch to time the etching process. 4. Etch the wafer for about half of the estimated time. Remove your sample, rinse with DI H20. 5. If the silicon dioxide has not been completely etched away, you will observe a film of the DI water wetting or sheeting across the whole surface of the wafer. If the layer of SiO2 has been etched off, the DI water will not adhere to the back, except for a few isolated drops, and the wafer will appear dry and dull gray. This happens because SiO2 is hydrophylic as opposed to silicon which is hydrophobic. Observe the backside of the wafer to confirm this effect for a few seconds. -

Hazardous Materials Safety Administration 49 CFR Parts 171, 172, 173, Et Al
Vol. 80 Thursday, No. 5 January 8, 2015 Part II Department of Transportation Pipeline and Hazardous Materials Safety Administration 49 CFR Parts 171, 172, 173, et al. Hazardous Materials: Harmonization With International Standards (RRR); Final Rule VerDate Sep<11>2014 19:02 Jan 07, 2015 Jkt 235001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\08JAR2.SGM 08JAR2 mstockstill on DSK4VPTVN1PROD with RULES2 1076 Federal Register / Vol. 80, No. 5 / Thursday, January 8, 2015 / Rules and Regulations DEPARTMENT OF TRANSPORTATION III. Incorporation by Reference Discussion reduces regulatory compliance costs and Under 1 CFR Part 51 helps to avoid costly frustrations of Pipeline and Hazardous Materials IV. Comment Discussion international shipments. PHMSA’s Safety Administration V. Section-by-Section Review continued leadership in maintaining VI. Regulatory Analyses and Notices A. Statutory/Legal Authority for the consistency with international 49 CFR Parts 171, 172, 173, 175, 176, Rulemaking regulations enhances the hazardous 178 and 180 B. Executive Orders 12866 and 13563 and materials safety program and assists in DOT Regulatory Policies and Procedures maintaining a favorable trade balance. [Docket Nos. PHMSA–2013–0260 (HM– C. Executive Order 13132 215M)] D. Executive Order 13175 II. Background E. Regulatory Flexibility Act, Executive RIN 2137–AF05 PHMSA published a notice of Order 13272, and DOT Policies and proposed rulemaking (NPRM) under Procedures Docket HM–215M (79 FR 50741, August Hazardous Materials: Harmonization F. Paperwork Reduction Act With International Standards (RRR) G. Regulatory Identifier Number (RIN) 25, 2014) to incorporate various H. Unfunded Mandates Reform Act amendments to harmonize the HMR AGENCY: Pipeline and Hazardous I. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Growth, Stability and Decomposition of Mg 2 Si Ultra-Thin Films on Si (100) Brice Sarpi, R Zirmi, Magali Putero, M Bouslama, Anne Hémeryck, Sébastien Vizzini
Growth, stability and decomposition of Mg 2 Si ultra-thin films on Si (100) Brice Sarpi, R Zirmi, Magali Putero, M Bouslama, Anne Hémeryck, Sébastien Vizzini To cite this version: Brice Sarpi, R Zirmi, Magali Putero, M Bouslama, Anne Hémeryck, et al.. Growth, stability and decomposition of Mg 2 Si ultra-thin films on Si (100). Applied Surface Science, Elsevier, 2018, 423 (Part B), pp.522-527. 10.1016/j.apsusc.2017.09.027. hal-01583845 HAL Id: hal-01583845 https://hal.laas.fr/hal-01583845 Submitted on 8 Sep 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Accepted Manuscript Title: Growth, stability and decomposition of Mg2Si ultra-thin films on Si (100) Authors: B. Sarpi, R. Zirmi, M. Putero, M. Bouslama, A. Hemeryck, S. Vizzini PII: S0169-4332(17)32642-9 DOI: http://dx.doi.org/10.1016/j.apsusc.2017.09.027 Reference: APSUSC 37106 To appear in: APSUSC Received date: 8-6-2017 Revised date: 4-9-2017 Accepted date: 5-9-2017 Please cite this article as: B.Sarpi, R.Zirmi, M.Putero, M.Bouslama, A.Hemeryck, S.Vizzini, Growth, stability and decomposition of Mg2Si ultra-thin films on Si (100), Applied Surface Sciencehttp://dx.doi.org/10.1016/j.apsusc.2017.09.027 This is a PDF file of an unedited manuscript that has been accepted for publication. -

Material Safety Data Sheet
Date of Issue: 21 September 2021 SAFETY DATA SHEET 1. SUBSTANCE AND SOURCE IDENTIFICATION Product Identifier SRM Number: 2557 SRM Name: Used Auto Catalyst (Monolith) Other Means of Identification: Not applicable. Recommended Use of This Material and Restrictions of Use This Standard Reference Material (SRM) is intended for use in evaluating chemical and instrumental methods for the analysis of platinum group metals and lead. A unit of SRM 2557 consists of a glass bottle containing 70 g of used auto catalyst as a fine powder [<74 µm (200 mesh)]. Company Information National Institute of Standards and Technology Standard Reference Materials Program 100 Bureau Drive, Stop 2300 Gaithersburg, Maryland 20899-2300 Telephone: 301-975-2200 Emergency Telephone ChemTrec: E-mail: [email protected] 1-800-424-9300 (North America) Website: http://www.nist.gov/srm +1-703-527-3887 (International) 2. HAZARDS IDENTIFICATION Classification Physical Hazard: Not classified. Health Hazard: Carcinogenicity Category 2 Label Elements Symbol Signal Word WARNING Hazard Statement(s): H351 Suspected of causing cancer (inhalation). Precautionary Statement(s): P201 Obtain special instructions before use. P202 Do not handle until all safety precautions have been read and understood. P281 Use personal protective equipment as required. P308+P313 If exposed or concerned: Get medical attention. P405 Store locked up. P501 Dispose of contents and container in accordance with local regulations. Hazards Not Otherwise Classified: Not applicable. Ingredients(s) with Unknown Acute Toxicity: Not applicable. SRM 2557 Page 1 of 7 3. COMPOSITION AND INFORMATION ON HAZARDOUS INGREDIENTS Substance: Used auto catalyst Other Designations: Recycled monolith auto catalyst This material is a complex mixture that has not been tested as a whole.