Igneous and Metamorphic Processes in the Shap Granite and Its Aureole
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

570 Bus Time Schedule & Line Route
570 bus time schedule & line map 570 Ravenstonedale - Kendal View In Website Mode The 570 bus line (Ravenstonedale - Kendal) has 2 routes. For regular weekdays, their operation hours are: (1) Kendal: 10:25 AM (2) Ravenstonedale: 2:55 PM Use the Moovit App to ƒnd the closest 570 bus station near you and ƒnd out when is the next 570 bus arriving. Direction: Kendal 570 bus Time Schedule 20 stops Kendal Route Timetable: VIEW LINE SCHEDULE Sunday Not Operational Monday Not Operational Classic Coaches Bus Depot, Ravenstonedale Tuesday Not Operational The Black Swan, Ravenstonedale Wednesday Not Operational Market Square, Shap Thursday 10:25 AM Main Street, Shap Civil Parish Friday Not Operational Woodville Terrace, Shap Green Croft, Shap Civil Parish Saturday Not Operational The Greyhound Hotel, Shap Brookƒeld B&B, Shap Brookƒeld, Shap Civil Parish 570 bus Info Direction: Kendal Turn For Shap Wells Hotel, Scout Green Stops: 20 Trip Duration: 90 min The Square, Orton Line Summary: Classic Coaches Bus Depot, Front Street, Orton Civil Parish Ravenstonedale, The Black Swan, Ravenstonedale, Market Square, Shap, Woodville Terrace, Shap, The Service Station, Old Tebay Greyhound Hotel, Shap, Brookƒeld B&B, Shap, Turn Orton Road, Tebay Civil Parish For Shap Wells Hotel, Scout Green, The Square, Orton, Service Station, Old Tebay, Mount Pleasant, Mount Pleasant, Tebay Tebay, Cross Keys, Tebay, Barnaby Rudge, Tebay, Primary School, Grayrigg, Crescent Green, Mintsfeet, Cross Keys, Tebay Morrisons, Mint Bridge, Lakeland Laundry, Mintsfeet, Victoria Apartments, -

Low Sadgill, Longsleddale
Low Sadgill, Longsleddale www.hackney-leigh.co.uk Low Sadgill Longsleddale Kendal Cumbria LA8 9BE £600,000 Low Sadgill is a splendid example of a late 16th/early 17th century Westmorland farmhouse with stone and slated outbuildings surrounded by approximately 2.3 acres of gardens, orchard and paddocks. There are four bedrooms, two bathrooms, a farmhouse kitchen, living room, study and music room with scope for more living space, workshops, garage and loft and a detached two storey outbuilding. Grade II listed this welcoming home is situated in an idyllic location surrounded by fields and fells at the head of one of Lakeland’s most attractive and delightfully unspoilt valleys, that offers perhaps a tiny piece of paradise in this all too busy world yet is just under ten miles from the bustling Market Town of Kendal. Description: Low Sadgill, is a Grade II listed former Westmorland farmhouse that started life as a packhorse station dating from possibly the 16th/17th century and is now offered for sale for someone new to enjoy its very special character and location at the head of the delightful valley of Longsleddale. The current layout is generous and flexible offering plenty of space to live, work or play, yet with scope to create further living space if required. Attention to detail has linked old and new to provide 21st Century comforts including secondary glazing to windows and the installation of oil central heating, all without interfering with period character, and many original features have been retained with exposed timbers and 17th Century oak doors with latch handles. -

The Westmorland Way
THE WESTMORLAND WAY WALKING IN THE HEART OF THE LAKES THE WESTMORLAND WAY - SELF GUIDED WALKING HOLIDAY SUMMARY The Westmorland Way is an outstanding walk from the Pennines, through the heart of the Lake District and to the Cumbrian Coast visiting the scenic and historical highlights of the old county of Westmorland. Your walk begins in Appleby-in-Westmorland which lies in the sandstone hills of the Pennines. It then heads west into the Lake District National Park, where you spend five unforgettable days walking through the heart of the Lake District. A final day of walking brings you to Arnside on Morecambe Bay. Along the way you will enjoy some of the Lake District’s most delightful landscapes, villages and paths. Ullswater, Windermere, Elterwater, Grasmere, Patterdale, Askham, Great Asby and Troutbeck all feature on your route through the lakes. Exploring the old county of Westmorland’s unparalleled variety is what makes this walk so enjoyable. From lakeside walks to mountain paths and canal towpaths the seven sections of the Westmorland Way Tour: The Westmorland Way will keep you enthralled from beginning to end. Code: WESWW1 Our walking holidays on the Westmorland Way include hand-picked overnight accommodation in high Type: Self-Guided Walking Holiday quality B&B’s, country inns, and guesthouses. Each is unique and offers the highest levels of welcome, Price: See Website atmosphere and outstanding local cuisine. We also include daily door to door baggage transfers, a Single Supplement: See Website Dates: April - October guidebook, detailed maps and a comprehensive pre-departure information pack as well as emergency Walking Days: 7 support, should you need it. -

Price £625000 Castle Court Crosby Ravensworth, Cumbria, CA10
Castle Court Crosby Ravensworth, Cumbria, CA10 3LG • Large Barn Conversion in a Stunning Rural Location • Over 3,500 Sq Ft Living Space + Adjoining 2 Storey Granary • Living Room, Dining Hall, Day Room, Farmhouse Kitchen + Garden Room • 5 Double Bedrooms, En-Suite Bathroom + 2 House Bathrooms • Laundry Room, Larder, Boot Room + 2 Cloakrooms • Fabulous Rural Position with Exceptional Open Views • Set in Approximately 1 Acre with a Range of Outbuildings • Mains Gas Central Heating + Open Fireplace and Multi Fuel Stove • EPC D Price £625,000 Set amidst beautiful open countryside with stunning views, Castle Court is a most impressive barn conversion over 3 floors with spacious, stylish and flexible accommodation comprising: Entrance Lobby, Dining Hall, Living Room, Guest Suite/Office with En-Suite Bathroom, Boot Room and Cloakroom all on the Ground Floor. A Day Room, Farmhouse Kitchen, Garden Room, Laundry, Cloakroom + Larder to the Basement level and on the upper floor there is a large Landing, 4 Double Bedrooms and 2 Bathrooms. Adjoining the barn is a 2 storey Granary which has planning permission for further accommodation if needed. Within the grounds is a range of Agricultural Outbuildings and a Stone Outhouse. Castle Court also benefits from mains gas central heating via a condensing Worcester boiler as well as having a multi fuel stove in the dining hall and an open fireplace in the living room. Location From Penrith head South on the A6 and drive to Shap. In Shap, turn left by the "Shap Chippy", signposted to Crosby Ravensworth. Drive for approximately 1.5 miles. Castle Court is on the left. -

1891 Census of Kentmere Cens Property Name Age Relationship Occupation Place of Birth No
1891 Census of Kentmere Cens Property Name Age Relationship Occupation Place of birth No. Name 1 Mags Howe Christopher Gilpin 73 Head Farmer 206 Acres Westmorland – Kentmere Emma 67 Wife Farmer’s Wife Westmorland – Kentmere Christopher 15 G-Son Scholar Westmorland - Kentmere 2 Brockstone James C Hindson 39 Head Farmer Westmorland – Shap Mary 34 Wife Farmer’s Wife Lancashire – Burton Ruth 6 Daughter Scholar Westm’d – Underbarrow Mary 5 Daughter Scholar Westm’d – Underbarrow Margaret 3 Daughter Westm’d – Underbarrow James C 2 Son Westmorland – Kentmere Emily 1 Daughter Westmorland – Kentmere Edith 6mo Daughter Westmorland – Kentmere Thomas Harrison 21 Servant Shepherd Westmorland – Bowness Robert J Kitching 22 Servant Farm Servant Westmorland – Sedgwick Mary E Rogers 24 Servant General Servant Domestic Cumberland - Penrith Jane Airey 55 Servant (W) Nurse Westmorland - Kentmere 3 Hallow Thomas Thompson 70 Head Farmer Westm’d – Longsleddale Bank Isabella 69 Wife Westmorland – Kentmere 4 Hallow James Airey 85 Head (W) Retired farmer Westmorland – Kentmere Bank Cott. Joseph 61 Son (W) Retired farmer Westm’d – Longsleddale Cicely 26 Servant General Servant Domestic Westmorland – Kentmere 5 The Howe James Walker 57 Head Slate Maker Westmorland – Kentmere Elizabeth 37 Wife Cumberland – Kirkoswald Stephen A 12 Son Scholar Westmorland – Kentmere Henry L 11 Son Scholar Westmorland – Kentmere Lizzie M 9 Daughter Scholar Westmorland - Kentmere 6 The Howe Thomas Storey 47 Head Slate Quarry Labourer Westmorland – Troutbeck Mary 45 Wife Lancashire – Coniston -

Metamorphic Effects on Agate Found Near the Shap Granite, Cumbria, England: As Demonstrated by Petrography, X-Ray Diffraction and Spectroscopic Methods
Mineralogical Magazine, August 2007, Vol. 71(4), pp. 461–476 Metamorphic effects on agate found near the Shap granite, Cumbria, England: as demonstrated by petrography, X-ray diffraction and spectroscopic methods 1, 2 2 T. MOXON *, S. J. B. REED AND M. ZHANG 1 55 Common Lane, Auckley, Doncaster DN9 3HX, UK 2 Department of Earth Sciences, University of Cambridge, Downing Street, Cambridge CB2 3EQ, UK [Received 18 June 2007; Accepted 18 December 2007] ABSTRACT Agates from a 430 Ma host at Stockdale Beck, Cumbria, England have been characterized. The crystallite size of the Stockdale Beck agates was found to be ~60% greater than any other agates from five regions aged 400À1100 Ma. Raman spectroscopy identified moganite in all agates tested except those from Stockdale Beck. Infrared spectroscopy showed that the silanol content of the Stockdale Beck agates was near zero. The properties of agates from Stockdale Beck and the 1.84À3.48 Ga metamorphosed hosts found in Western Australia were similar but different from agates found in other hosts aged 400À1100 Ma. Cathodoluminescence demonstrates further differences between agates from hosts aged 13À1100 Ma and those from Stockdale Beck and Western Australia. Agates from the latter areas have a lower proportion of defects causing a red emission band (~660 nm) but an increased proportion of defects causing blue (~470 nm) and orange (~640 nm) emission bands. Agates found in hosts aged 13À1100 Ma are also differentiated from the Stockdale Beck and Western Australian agates in a ternary plot of the relative intensities of violet to blue to orange emission bands. -

North West Geography
ISSN 1476-1580 North West Geography Volume 14, Number 1, 2014 North West Geography, Volume 14, 2014 12 Towards a robust deglacial chronology for the northwest England sector of the last British-Irish Ice Sheet Peter Wilson Environmental Sciences Research Institute, School of Environmental Sciences, University of Ulster, Coleraine, Co. Londonderry BT52 1SA. [email protected] Tom Lord Lower Winskill, Langcliffe, Settle, North Yorkshire BD24 9PZ. Abstract A number of absolute age determinations that provide a timeframe for the deglaciation of the last ice sheet in northwest England are reviewed. Some of the ages are probably too old and are therefore unreliable; some others have large associated uncertainties and are imprecise estimates for the loss of ice cover. Several ages are minimum ages for deglaciation because they record the timing of sedimentary events made possible by the removal of ice. The tightest age constraints on deglaciation are those derived from cosmogenic nuclide surface exposure dating but for some sites only a single age is available. Nevertheless together these age determinations indicate that between ~18 ka and ~17 ka northwest England began to emerge from its cover of glacial ice. Valley glaciers persisted in the Lake District until ~15 ka but had probably disappeared by 14.7 ka, or shortly after, when climate warmed abruptly. A more detailed picture of the style and rate of deglaciation is likely to come in the next few years as a result of the BRITICE-CHRONO project. Keywords Deglaciation, Last Glacial Maximum, British-Irish Ice Sheet, Dating techniques, Northwest England. Introduction Interest in the glacial geomorphology and sediment The idea that glaciers had previously existed in the British stratigraphy of northwest England extends back to the and Irish Isles was proposed by Swiss geologist Louis earliest days of glacial study. -

E D E N G R O
E D E N G R O V E APPLEBY-IN-WESTMORLAND CUMBRIA A BEAUTIFUL DEVELOPMENT OF LUXURY HOMES AND APARTMENTS SITUATED IN THE PICTURESQUE VILLAGE OF BOLTON Location, location, location... IMAGINE A PLACE, A PLACE SO BEAUTIFUL THAT YOU WISH YOU COULD SPEND EVERY DAY THERE. A PLACE WITH CHARACTER AND CHARM BUT MOST OF ALL; A PLACE YOU COULD CALL HOME. OUR LATEST DEVELOPMENT NESTLED IN THE EDEN VALLEY EMBRACES IT ALL. RIVER EDEN - BY LOCAL PHOTOGRAPHER RIC HALL Apartments 1-4 Derwent House Aerial Site Plan Apartments 1-4 Buttermere House Development Address: Laurel Mount Eden Grove Apartments 1-8 Eden Grove Bolton A development like no other... Appleby-in-Westmorland CA16 6FT / CA16 6FS Number 8 WHEN WE PLAN A NEW DEVELOPMENT, WE ALWAYS AIM TO Number 4 PROVIDE A RANGE OF HOUSE TYPES THAT WILL SUIT MOST PEOPLE’S CHANGING NEEDS. Number 6 HERE AT EDEN GROVE, WE HAVE COVERED EVERYTHING. 1 & 2 Blencathra House Number 2 FROM ONE BEDROOM APARTMENTS, THROUGH TO FOUR BEDROOM Laurel Mount HOMES (BEING RELEASED IN PHASE 2), YOU WILL BE SPOILT FOR CHOICE. ALL SET WITHIN THE GROUNDS OF THE FORMER EDEN GROVE RESIDENTIAL SCHOOL, YOU’LL FIND A MATURITY NOT NORMALLY FOUND ON MOST DEVELOPMENTS. COMPUTER GENERATED IMAGE SHOWING NUMBER 1 LAUREL MOUNT WITH A TASTEFUL CONVERSION TO THE ORIGINAL SCHOOL BUILDINGS THAT OFFER TRADITIONAL ELEGANCE ON THE OUTSIDE, 1 & 2 Coniston House COMPLEMENTED BY CONTEMPORARY LUXURY ON THE INSIDE, OUR APARTMENTS ARE DESIGNED AND RENOVATED TO THE HIGHEST STANDARDS TO EMPATHISE WITH THE EVER-CHANGING REQUIREMENTS OF MODERN LIVING. -

Landscape and Visual Impact Appraisals Appleby-In-Westmorland PDP Associates Landscape Architects
Eden District Council Housing Development Plan Landscape and Visual Impact Appraisals Appleby-in-Westmorland PDP Associates Landscape Architects CONTENTS Section 1: Introduction Page 2 1.1 The proposed sites Section 2: Methodology Page 4 2.1 Landscape effects 2.2 Magnitude of landscape impacts 2.3 Visual effects 2.4 Sensitivity of viewpoints 2.5 Magnitude of visual impacts 2.6 Terminology 2.7 Key issues Section 3: Policy context/framework Page 10 3.1 Policy guidance 3.2 The landscape setting 3.3 General descriptions of the sites Section 4: Landscape and visual impacts and their significance Page 19 4.1 Visual baseline 4.2 Landscape sensitivity of each parcel 4.3 Summary of landscape impacts 4.4 Visual impact of each parcel 4.5 Summary of visual impacts Section 5: Summary Page 35 5.1 Summary of parcel AP5 5.2 Summary of Parcels AP6 & AP16 5.3 Summary of Parcel AP10 5.4 Summary of Parcel AP11 5.5 Summary of Parcel AP12 5.6 Summary of Parcel AP14 Landscape and Visual Impact Appraisal Appleby in Westmorland 1 PDP Associates Landscape Architects 1.0 Introduction PDP Associates has been instructed by Eden District Council to undertake landscape and visual impact appraisals on various sites in Appleby in Westmorland. This information will inform the Housing Development Plan by helping to assess individual site’s suitability for incorporating housing (landscape impact), and the impact any such development might have on the wider area (visual impact). By following a structured assessment method, it has been possible to rank each site according to its overall suitability for use for housing. -
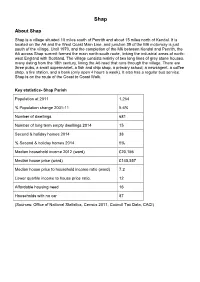
Settlement Profile: Shap
Shap About Shap Shap is a village situated 10 miles south of Penrith and about 15 miles north of Kendal. It is located on the A6 and the West Coast Main Line, and junction 39 of the M6 motorway is just south of the village. Until 1970, and the completion of the M6 between Kendal and Penrith, the A6 across Shap summit formed the main north-south route, linking the industrial areas of north- west England with Scotland. The village consists mainly of two long lines of grey stone houses, many dating from the 18th century, lining the A6 road that runs through the village. There are three pubs, a small supermarket, a fish and chip shop, a primary school, a newsagent, a coffee shop, a fire station, and a bank (only open 4 hours a week). It also has a regular bus service. Shap is on the route of the Coast to Coast Walk. Key statistics- Shap Parish Population at 2011 1,264 % Population change 2001-11 5.6% Number of dwellings 681 Number of long term empty dwellings 2014 15 Second & holiday homes 2014 38 % Second & holiday homes 2014 5% Median household income 2012 (ward) £20,156 Median house price (ward) £145,557 Median house price to household income ratio (ward) 7.2 Lower quartile income to house price ratio. 12 Affordable housing need 16 Households with no car 87 (Sources: Office of National Statistics, Census 2011, Council Tax Data, CACI) Map showing sites in Shap Blue boundary - Preferred sites proposed for allocation Red boundary - Sites assessed and not proposed for allocation ©Crown Copyright and Database Rights (100023754) (2014) LSH1 - West Lane, Shap LSH1 - West Lane, Shap Is this site proposed for Yes development? Size 0.27 hectares Potential Number of Houses 8 Brownfield? No Description The site is a greenfield site. -

Longsleddale Parish Plan
Longsleddale Parish Plan Contents Page 1. Introduction 2 1.1 The place 2 1.2 The people 3 1.3 Drawing up the plan 3 2. The Plan 5 2.1 Vision 5 2.2 Planning 6 2.3 Community and Visitors 7 2.4 Roads, verges, hedges and tracks 7 2.5 Transport 8 2.6 Electronic Communications 9 2.7 Energy 9 2.8 Other Services 10 2.8.1 Emergency services 10 2.8.2 Health 10 2.8.3 Education 10 2.8.4 Refuse 11 2.8.5 Post 11 2.9 Wildlife 11 1 Longsleddale Parish Plan 1. Introduction 1.1 The Place Longsleddale is a parish in the ward of Whinfell, the district of South Lakeland, the county of Cumbria, and it is in the Lake District National Park. It is bounded by the parishes of Over Staveley and Kentmere to the west; Shap Rural to the north; Fawcett Forest and Whitwell & Selside to the east; Strickland Roger to the south. The parish is an area of 2717 hectares, encompassing the upper valley of the River Sprint, 8 miles long, 2 miles wide, ranging from SD5299 to NY4607, from Garnett Bridge in the south to Harter Fell in the north. Longsleddale is a non-nucleated village, the 30 houses being scattered over 4½ miles, each sited above the flood plain, but where there is water from spring or stream all year round. The centre of the community, with Church and Community Hall, is at NY500029. Longsleddale has no parish council, but has a well attended Parish Meeting twice a year. -

High House LONGSLEDDALE, KENDAL
High House LONGSLEDDALE, KENDAL www.jackson-stops.co.uk Description High House is set 6 1/2 miles North of Kendal town centre in a breathtaking valley amidst some of the county’s finest countryside. The property is not only a fine family house, but with its guest annexe lends itself for the use as a holiday cottage. Constructed of stone under a slate roof, the house dates back to the mid 17th century and enjoys a South Westerly aspect looking across the valley in beautifully landscaped gardens. Upon first entering the house there is a large living dining room with a fireplace incorporating a wood burner, access to a study and a delightful breakfast kitchen with a range of fitted units. Stepping down from the kitchen area is a utility room, pantry and shower room. To the first floor from the main landing four of the bedrooms can be accessed (one en suite) and the family bathroom with one of the bedrooms having a mezzanine study area. Just off bedroom four access to the guest annexe can be found which also enjoys direct external access. The annexe comes complete with a fully fitted kitchen and galleried bedroom. There are a wealth of features within the property including open fires, exposed beams, latch and brace doors complimented by the more modern day convenience of double glazing and an oil fired central heating system. A charming mid Accommodation in Brief 17th Century farm • Living Dining Room • Study • Breakfast Kitchen • Utility Room house set in unspoilt • Pantry • Shower Room • Five Bedrooms • Two Bathrooms • Galleried Study • Landscaped Gardens lakeland valley • Guest Annexe with Second Kitchen • Large Boarded Attic Storage • Lease of a Detached Barn for Garaging Purposes Outside Situated in a slightly elevated position amidst delightful landscaped gardens, High House sits well within its plot and enjoys a driveway with ample parking and turning space.