An Australopithecus Afarensis Infant First Metatarsal from Hadar, Ethiopia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geologists Probe Hominid Environments
1999 PRESIDENTIAL ADDRESS Geologists Probe Hominid Environments Gail M. Ashley, Department of Geological Sciences, Rutgers University, New Brunswick, NJ 08903, USA, [email protected] ABSTRACT challenging areas of research often lie at artificially imposed disci- pline boundaries. Here lies the potential for synergy and perhaps The study of an early Pleistocene “time slice” in Olduvai even the generation of a new science (Fig. 2). However, integrat- Gorge, Tanzania, provides a successful example of a recon- ing sciences is not as easy as it might first appear. It requires peo- structed paleolandscape that is rich in detail and adds a small ple to learn language, theories, methodologies, and a bit about piece to the puzzle of hominid evolution in Africa. The recon- the “culture” of the other science and to continually walk in the struction required multidisciplinary interaction of sedimen- other person’s shoes. Simply having lots of scientists with differ- tologists, paleoanthropologists, paleoecologists, and geochro- ent backgrounds working in parallel on the same project doesn’t nologists. Geology plays an increasingly important role in produce the same end result as integrative science. unraveling the record of hominid evolution. Key questions This paper describes a study at Olduvai Gorge, Tanzania (Fig. regarding paleoclimate, paleoenvironment, and perhaps even 3), using a relatively new approach, landscape paleoanthropol- hominid land use are answered by geology, and these answers ogy, that attempts to interpret the landscape during a geologic provide a basis for multidisciplinary work. Landscape pale- instant in time. The project is the Olduvai Landscape Paleo- oanthropology integrates these data from several disciplines anthropology Project (OLAPP), involving a multidisciplinary to interpret the ecological context of hominids during a nar- team. -

Homo Habilis
COMMENT SUSTAINABILITY Citizens and POLICY End the bureaucracy THEATRE Shakespeare’s ENVIRONMENT James Lovelock businesses must track that is holding back science world was steeped in on surprisingly optimistic governments’ progress p.33 in India p.36 practical discovery p.39 form p.41 The foot of the apeman that palaeo ‘handy man’, anthropologists had been Homo habilis. recovering in southern Africa since the 1920s. This, the thinking went, was replaced by the taller, larger-brained Homo erectus from Asia, which spread to Europe and evolved into Nean derthals, which evolved into Homo sapiens. But what lay between the australopiths and H. erectus, the first known human? BETTING ON AFRICA Until the 1960s, H. erectus had been found only in Asia. But when primitive stone-chop LIBRARY PICTURE EVANS MUSEUM/MARY HISTORY NATURAL ping tools were uncovered at Olduvai Gorge in Tanzania, Leakey became convinced that this is where he would find the earliest stone- tool makers, who he assumed would belong to our genus. Maybe, like the australopiths, our human ancestors also originated in Africa. In 1931, Leakey began intensive prospect ing and excavation at Olduvai Gorge, 33 years before he announced the new human species. Now tourists travel to Olduvai on paved roads in air-conditioned buses; in the 1930s in the rainy season, the journey from Nairobi could take weeks. The ravines at Olduvai offered unparalleled access to ancient strata, but field work was no picnic in the park. Water was often scarce. Leakey and his team had to learn to share Olduvai with all of the wild animals that lived there, lions included. -
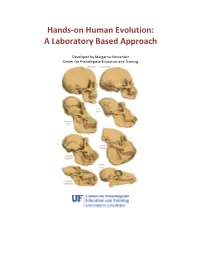
Hands-On Human Evolution: a Laboratory Based Approach
Hands-on Human Evolution: A Laboratory Based Approach Developed by Margarita Hernandez Center for Precollegiate Education and Training Author: Margarita Hernandez Curriculum Team: Julie Bokor, Sven Engling A huge thank you to….. Contents: 4. Author’s note 5. Introduction 6. Tips about the curriculum 8. Lesson Summaries 9. Lesson Sequencing Guide 10. Vocabulary 11. Next Generation Sunshine State Standards- Science 12. Background information 13. Lessons 122. Resources 123. Content Assessment 129. Content Area Expert Evaluation 131. Teacher Feedback Form 134. Student Feedback Form Lesson 1: Hominid Evolution Lab 19. Lesson 1 . Student Lab Pages . Student Lab Key . Human Evolution Phylogeny . Lab Station Numbers . Skeletal Pictures Lesson 2: Chromosomal Comparison Lab 48. Lesson 2 . Student Activity Pages . Student Lab Key Lesson 3: Naledi Jigsaw 77. Lesson 3 Author’s note Introduction Page The validity and importance of the theory of biological evolution runs strong throughout the topic of biology. Evolution serves as a foundation to many biological concepts by tying together the different tenants of biology, like ecology, anatomy, genetics, zoology, and taxonomy. It is for this reason that evolution plays a prominent role in the state and national standards and deserves thorough coverage in a classroom. A prime example of evolution can be seen in our own ancestral history, and this unit provides students with an excellent opportunity to consider the multiple lines of evidence that support hominid evolution. By allowing students the chance to uncover the supporting evidence for evolution themselves, they discover the ways the theory of evolution is supported by multiple sources. It is our hope that the opportunity to handle our ancestors’ bone casts and examine real molecular data, in an inquiry based environment, will pique the interest of students, ultimately leading them to conclude that the evidence they have gathered thoroughly supports the theory of evolution. -

Orangutan…Taxonomy…And…Nomenclature
«««« ORANGUTAN…TAXONOMY…AND…NOMENCLATURE« « Craig«D em itros« « The«taxonom y«of«the«orangutan«has«been«confusing«and«is«still«the«subject«of« m uch«debate.«Q uestions«at«the«specific«and«subspecific«level«are«still«being« investigated«(Courtenay«et«al.«1988).«The«follow ing«taxonom ic«inform ation«is« taken«prim arily«from «G roves,«1971.« « H IG H ER«LEVEL«TAXO N O M Y:« O rder:«Prim ates« Suborder:«A nthropoidea« Superfam ily:«H om inoidea« Fam ily:«Pongidae«(Includes«extant«genera«Pan,…Gorilla…and…Pongo).« « H ISTO RICA L«TAXO N O M Y«AT«TH E«G EN U S«A N D «G EN U S«SPECIES«LEVEL:«« G enus« Pongo«Lacepede,«1799.« O urangus«Zim m erm an,«1777«(N am e«invalidated).« « G enus«species«(Pongo…pygm aeus«H oppius,«1763).« Sim ia…pygm aeus«H oppius,«1763.««Type«locality«Sum atra.« Sim ia…satyrus«Linnaeus,«1766.« O urangus…outangus«Zim m erm an,«1777.« Pongo…borneo«Lacepede,«1799.««Type«locality«Borneo.« Sim ia…Agrais«Schreber,«1779.««Type«locality«Borneo.« Pongo…W urm bii«Tiedem ann,«1808.««Type«locality«Borneo.« Pongo…Abelii«Lesson,«1827.««Type«locality«Sum atra.« Sim ia…M orio«O w en,«1836.««Type«locality«Borneo.« Pithecus…bicolor«I.«G eoffroy,«1841.««Type«locality«Sum atra.« Sim ia…Gargantica«Pearson,«1841.««Type«locality«Sum atra.« Pithecus…brookei«Blyth,«1853.««Type«locality«Saraw ak.« Pithecus…ow enii«Blyth,«1853.««Type«locality«Saraw ak.« Pithecus…curtus«Blyth,«1855.««Type«locality«Saraw ak.« Satyrus…Knekias«M eyer,«1856.««Type«locality«Borneo.« Pithecus…W allichii«G ray,«1870.««Type«locality«Borneo.« Pithecus…sum atranus«Selenka,«1896.««Type«locality«Sum atra.« Pongo…pygm aeus«Rothschild,«1904.««First«use«of«this«com bination.« Ptihecus…w allacei«Elliot,«1913.««Type«locality«Borneo.« « CURRENT…TAXONOMY« « The«current«and«m ost«accepted«taxonom y«of«the«G enus«Pongo«includes«one« species«Pongo…pygm aeus«and«tw o«subspecies«P.p.…pygm aeus«(the«Bornean« subspecies)«and«P.p.…abelii«(the«Sum atran«subspecies)«(Bem m el«1968;«Jones« 1969;«G roves«1971;«Jacobshagen«1979;«Seuarez«et«al.«1979«and«G roves«1993).« 5« « . -

Neither Chimpanzee Nor Human, Ardipithecus Reveals the Surprising Ancestry of Both Tim D
SPECIAL FEATURE: PERSPECTIVE PERSPECTIVE SPECIAL FEATURE: Neither chimpanzee nor human, Ardipithecus reveals the surprising ancestry of both Tim D. Whitea,1, C. Owen Lovejoyb, Berhane Asfawc, Joshua P. Carlsona, and Gen Suwad,1 aDepartment of Integrative Biology, Human Evolution Research Center, University of California, Berkeley, CA 94720; bDepartment of Anthropology, School of Biomedical Sciences, Kent State University, Kent, OH 44242–0001; cRift Valley Research Service, Addis Ababa, Ethiopia; and dThe University Museum, The University of Tokyo, Hongo, Bunkyo-ku Tokyo 113-0033, Japan Edited by Neil H. Shubin, University of Chicago, Chicago, IL, and approved September 10, 2014 (received for review April 25, 2014) Australopithecus fossils were regularly interpreted during the late 20th century in a framework that used living African apes, especially chimpanzees, as proxies for the immediate ancestors of the human clade. Such projection is now largely nullified by the discovery of Ardipithecus. In the context of accumulating evidence from genetics, developmental biology, anatomy, ecology, biogeography, and geology, Ardipithecus alters perspectives on how our earliest hominid ancestors—and our closest living relatives—evolved. human evolution | Australopithecus | hominid | Ethiopia “...the stock whence two or more species have chimpanzees, can serve as adequate repre- (5). Indeed, a widely used textbook still pro- sprung, need in no respect be intermediate sentations of the ancestral past. claims that, “Overall, Au. afarensis seems very between those species.” much like a missing link between the living Background T. H. Huxley, 1860 (1) Africanapesandlaterhomininsinitsdental, ’ Darwin s human evolution scenario attemp- cranial, and skeletal morphology” (6). Charles Darwin famously suggested that ted to explain hominid tool use, bipedality, Australopithecus can no longer be legiti- Africa was humanity’s most probable birth enlarged brains, and reduced canine teeth (2). -

Studbook Gibbons 07
European Studbook Number 2 (data 31.12.2006) Edited by Pierre Moisson & Mélanie Berthet Northern White-cheeked Gibbon - Nomascus leucogenys Southern White-cheeked Gibbon - Nomascus siki Red-cheeked Gibbon - Nomascus gabriellae With Nutrition guidelines by David Gomis and a summary of Hylobatidae diseases 1 Nutrition guidelines for “Concolor” gibbons by David Gomis, DVM, with the collaboration of Sara De Michelis, PhD ; Thijs Flahou, DVM ; Lise Turner, DVM. These nutritional guidelines can also be used for other Hylobatidae species, except perhaps Siamangs. Part of this work was undertaken in 2005 by L. Turner for her veterinary thesis (Cf. 9- Ref. 84) and more recently in 2006-2007 with T. Flahou for the Mulhouse Zoo Dietary Manual publication. 1- Introduction: The present guidelines have been written in response to a lack of research and published informations on Nomascus subspecies diets. Meeting the nutritional needs of gibbons is essential to assure their survival and their reproduction in captivity. Present guidelines are not nutrition recommendations, but a first evaluation done in Mulhouse Zoo. Our Zoo has experience with keeping and breeding gibbons since 1961, and even if their nutrition doesn’t seem to represent a real difficulty compared to other non human primates, the diets have been improved over these 46 years. Therefore this work does not pretend to be exhaustive. Hopefully it would initiate some more nutrition research and coordination among zoos, with the objective of improving the database. Zoo animal nutrition is increasingly being recognised as a specialty: knowledge available is increasing too. The first aim of this study was to provide a database, useful for the development of diets for “Concolor” gibbons, as objectively as possible: with this purpose, we synthetized the few data we could collect on “Concolor” gibbons’ diets and nutrient requirements. -

Human Evolution: a Paleoanthropological Perspective - F.H
PHYSICAL (BIOLOGICAL) ANTHROPOLOGY - Human Evolution: A Paleoanthropological Perspective - F.H. Smith HUMAN EVOLUTION: A PALEOANTHROPOLOGICAL PERSPECTIVE F.H. Smith Department of Anthropology, Loyola University Chicago, USA Keywords: Human evolution, Miocene apes, Sahelanthropus, australopithecines, Australopithecus afarensis, cladogenesis, robust australopithecines, early Homo, Homo erectus, Homo heidelbergensis, Australopithecus africanus/Australopithecus garhi, mitochondrial DNA, homology, Neandertals, modern human origins, African Transitional Group. Contents 1. Introduction 2. Reconstructing Biological History: The Relationship of Humans and Apes 3. The Human Fossil Record: Basal Hominins 4. The Earliest Definite Hominins: The Australopithecines 5. Early Australopithecines as Primitive Humans 6. The Australopithecine Radiation 7. Origin and Evolution of the Genus Homo 8. Explaining Early Hominin Evolution: Controversy and the Documentation- Explanation Controversy 9. Early Homo erectus in East Africa and the Initial Radiation of Homo 10. After Homo erectus: The Middle Range of the Evolution of the Genus Homo 11. Neandertals and Late Archaics from Africa and Asia: The Hominin World before Modernity 12. The Origin of Modern Humans 13. Closing Perspective Glossary Bibliography Biographical Sketch Summary UNESCO – EOLSS The basic course of human biological history is well represented by the existing fossil record, although there is considerable debate on the details of that history. This review details both what is firmly understood (first echelon issues) and what is contentious concerning humanSAMPLE evolution. Most of the coCHAPTERSntention actually concerns the details (second echelon issues) of human evolution rather than the fundamental issues. For example, both anatomical and molecular evidence on living (extant) hominoids (apes and humans) suggests the close relationship of African great apes and humans (hominins). That relationship is demonstrated by the existing hominoid fossil record, including that of early hominins. -

Paranthropus Boisei: Fifty Years of Evidence and Analysis Bernard A
Marshall University Marshall Digital Scholar Biological Sciences Faculty Research Biological Sciences Fall 11-28-2007 Paranthropus boisei: Fifty Years of Evidence and Analysis Bernard A. Wood George Washington University Paul J. Constantino Biological Sciences, [email protected] Follow this and additional works at: http://mds.marshall.edu/bio_sciences_faculty Part of the Biological and Physical Anthropology Commons Recommended Citation Wood B and Constantino P. Paranthropus boisei: Fifty years of evidence and analysis. Yearbook of Physical Anthropology 50:106-132. This Article is brought to you for free and open access by the Biological Sciences at Marshall Digital Scholar. It has been accepted for inclusion in Biological Sciences Faculty Research by an authorized administrator of Marshall Digital Scholar. For more information, please contact [email protected], [email protected]. YEARBOOK OF PHYSICAL ANTHROPOLOGY 50:106–132 (2007) Paranthropus boisei: Fifty Years of Evidence and Analysis Bernard Wood* and Paul Constantino Center for the Advanced Study of Hominid Paleobiology, George Washington University, Washington, DC 20052 KEY WORDS Paranthropus; boisei; aethiopicus; human evolution; Africa ABSTRACT Paranthropus boisei is a hominin taxon ers can trace the evolution of metric and nonmetric var- with a distinctive cranial and dental morphology. Its iables across hundreds of thousands of years. This pa- hypodigm has been recovered from sites with good per is a detailed1 review of half a century’s worth of fos- stratigraphic and chronological control, and for some sil evidence and analysis of P. boi se i and traces how morphological regions, such as the mandible and the both its evolutionary history and our understanding of mandibular dentition, the samples are not only rela- its evolutionary history have evolved during the past tively well dated, but they are, by paleontological 50 years. -

Curren T Anthropology
Forthcoming Current Anthropology Wenner-Gren Symposium Curren Supplementary Issues (in order of appearance) t Humanness and Potentiality: Revisiting the Anthropological Object in the Anthropolog Current Context of New Medical Technologies. Klaus Hoeyer and Karen-Sue Taussig, eds. Alternative Pathways to Complexity: Evolutionary Trajectories in the Anthropology Middle Paleolithic and Middle Stone Age. Steven L. Kuhn and Erella Hovers, eds. y THE WENNER-GREN SYMPOSIUM SERIES Previously Published Supplementary Issues December 2012 HUMAN BIOLOGY AND THE ORIGINS OF HOMO Working Memory: Beyond Language and Symbolism. omas Wynn and Frederick L. Coolidge, eds. GUEST EDITORS: SUSAN ANTÓN AND LESLIE C. AIELLO Engaged Anthropology: Diversity and Dilemmas. Setha M. Low and Sally Early Homo: Who, When, and Where Engle Merry, eds. Environmental and Behavioral Evidence V Dental Evidence for the Reconstruction of Diet in African Early Homo olum Corporate Lives: New Perspectives on the Social Life of the Corporate Form. Body Size, Body Shape, and the Circumscription of the Genus Homo Damani Partridge, Marina Welker, and Rebecca Hardin, eds. Ecological Energetics in Early Homo e 5 Effects of Mortality, Subsistence, and Ecology on Human Adult Height 3 e Origins of Agriculture: New Data, New Ideas. T. Douglas Price and Plasticity in Human Life History Strategy Ofer Bar-Yosef, eds. Conditions for Evolution of Small Adult Body Size in Southern Africa Supplement Growth, Development, and Life History throughout the Evolution of Homo e Biological Anthropology of Living Human Populations: World Body Size, Size Variation, and Sexual Size Dimorphism in Early Homo Histories, National Styles, and International Networks. Susan Lindee and Ricardo Ventura Santos, eds. -

Identity of Newly Found, Fully Intact Hominid Skulls from Ethiopia Chris Lemke College of Dupage
ESSAI Volume 7 Article 31 4-1-2010 Identity of Newly Found, Fully Intact Hominid Skulls from Ethiopia Chris Lemke College of DuPage Follow this and additional works at: http://dc.cod.edu/essai Recommended Citation Lemke, Chris (2009) "Identity of Newly Found, Fully Intact Hominid Skulls from Ethiopia," ESSAI: Vol. 7, Article 31. Available at: http://dc.cod.edu/essai/vol7/iss1/31 This Selection is brought to you for free and open access by the College Publications at [email protected].. It has been accepted for inclusion in ESSAI by an authorized administrator of [email protected].. For more information, please contact [email protected]. Lemke: Identity of Hominid Skulls Identity of Newly Found, Fully Intact Hominid Skulls from Ethiopia by Chris Lemke (Honors Biology 1151) ABSTRACT ecently, three fully intact hominid skulls have been found in the Afar Region of Ethiopia. Objectives were to date the skulls using Uranium-235, and to identify each of the skulls. RUranium-235 dating indicated skulls A and B to be 2.9 million years old, and skull C to be 1.7 million years old. Each skull was properly identified using existing fossil data. The two oldest skulls were found to be Australopithecus afarensis, and A. africanus. The younger skull was identified as Homo habilis. A discrepancy was found in the measured cranial capacity data against existing data. Due to condition of the newly found fossils, the most likely explanation for the discrepancy is inaccuracy of existing fossil data due to incomplete and fragmented specimens, or that the skulls in question were representative of a juvenile hominid. -

Darwin and the Recent African Origin of Modern Humans
EDITORIAL Darwin and the recent African origin of modern humans Richard G. Klein1 Program in Human Biology, Stanford University, Stanford, CA 94305 n this 200th anniversary of When Darwin and Huxley were ac- The Course of Human Evolution Charles Darwin’s birth and tive, many respected scientists sub- In the absence of fossils, Darwin could the 150th anniversary of the scribed to the now discredited idea that not have predicted the fundamental pat- publication of his monumen- human races represented variably tern of human evolution, but his evolu- Otal The Origin of Species (1859) (1), it evolved populations of Homo sapiens. tionary theory readily accommodates seems fitting to summarize Darwin’s The original Neanderthal skull had a the pattern we now recognize. Probably views on human evolution and to show conspicuous browridge, and compared the most fundamental finding is that the how far we have come since. Darwin with the skulls of modern humans, it australopithecines, who existed from at famously neglected the subject in The was decidedly long and low. At the same least 4.5 million to 2 million years ago, Origin, except near the end where he time, it had a large braincase, and Hux- were distinguished from apes primarily noted only that ‘‘light would be thrown ley regarded it as ‘‘the extreme term of by anatomical specializations for habit- on the origin of man and his history’’ by a series leading gradually from it to the ual bipedalism, and it was only after 2 the massive evidence he had compiled highest and best developed of [modern] million years ago that people began to for evolution by means of natural selec- human crania.’’ It was only in 1891 that acquire the other traits, including our tion. -

Lieberman 2001E.Pdf
news and views Another face in our family tree Daniel E. Lieberman The evolutionary history of humans is complex and unresolved. It now looks set to be thrown into further confusion by the discovery of another species and genus, dated to 3.5 million years ago. ntil a few years ago, the evolutionary history of our species was thought to be Ureasonably straightforward. Only three diverse groups of hominins — species more closely related to humans than to chim- panzees — were known, namely Australo- pithecus, Paranthropus and Homo, the genus to which humans belong. Of these, Paran- MUSEUMS OF KENYA NATIONAL thropus and Homo were presumed to have evolved between two and three million years ago1,2 from an early species in the genus Australopithecus, most likely A. afarensis, made famous by the fossil Lucy. But lately, confusion has been sown in the human evolutionary tree. The discovery of three new australopithecine species — A. anamensis3, A. garhi 4 and A. bahrelghazali5, in Kenya, Ethiopia and Chad, respectively — showed that genus to be more diverse and Figure 1 Two fossil skulls from early hominin species. Left, KNM-WT 40000. This newly discovered widespread than had been thought. Then fossil is described by Leakey et al.8. It is judged to represent a new species, Kenyanthropus platyops. there was the finding of another, as yet poorly Right, KNM-ER 1470. This skull was formerly attributed to Homo rudolfensis1, but might best be understood, genus of early hominin, Ardi- reassigned to the genus Kenyanthropus — the two skulls share many similarities, such as the flatness pithecus, which is dated to 4.4 million years of the face and the shape of the brow.