Understanding Root Systems to Improve, Seedling Quality
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plant Physiology
PLANT PHYSIOLOGY Vince Ördög Created by XMLmind XSL-FO Converter. PLANT PHYSIOLOGY Vince Ördög Publication date 2011 Created by XMLmind XSL-FO Converter. Table of Contents Cover .................................................................................................................................................. v 1. Preface ............................................................................................................................................ 1 2. Water and nutrients in plant ............................................................................................................ 2 1. Water balance of plant .......................................................................................................... 2 1.1. Water potential ......................................................................................................... 3 1.2. Absorption by roots .................................................................................................. 6 1.3. Transport through the xylem .................................................................................... 8 1.4. Transpiration ............................................................................................................. 9 1.5. Plant water status .................................................................................................... 11 1.6. Influence of extreme water supply .......................................................................... 12 2. Nutrient supply of plant ..................................................................................................... -

What Are Soybeans?
candy, cakes, cheeses, peanut butter, animal feeds, candles, paint, body lotions, biodiesel, furniture soybeans USES: What are soybeans? Soybeans are small round seeds, each with a tiny hilum (small brown spot). They are made up of three basic parts. Each soybean has a seed coat (outside cover that protects the seed), VOCABULARY cotyledon (the first leaf or pair of leaves within the embryo that stores food), and the embryo (part of a seed that develops into Cultivar: a variety of plant that has been created or a new plant, including the stem, leaves and roots). Soybeans, selected intentionally and maintained through cultivation. like most legumes, perform nitrogen fixation. Modern soybean Embryo: part of a seed that develops into a new plant, cultivars generally reach a height of around 1 m (3.3 ft), and including the stem, leaves and roots. take 80–120 days from sowing to harvesting. Exports: products or items that the U.S. sells and sends to other countries. Exports include raw products like whole soybeans or processed products like soybean oil or Leaflets soybean meal. Fertilizer: any substance used to fertilize the soil, especially a commercial or chemical manure. Hilum: the scar on a seed marking the point of attachment to its seed vessel (the brown spot). Leaflets: sub-part of leaf blade. All but the first node of soybean plants produce leaves with three leaflets. Legume: plants that perform nitrogen fixation and whose fruit is a seed pod. Beans, peas, clover and alfalfa are all legumes. Nitrogen Fixation: the conversion of atmospheric nitrogen Leaf into a nitrogen compound by certain bacteria, such as Stem rhizobium in the root nodules of legumes. -

International Union for the Protection of New Varieties of Plants
E TG/81/7(proj.1) ORIGINAL: English DATE: 2018-04-05 INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS Geneva DRAFT * COMMON SUNFLOWER UPOV Code(s): HLNTS_ANN Helianthus annuus L. GUIDELINES FOR THE CONDUCT OF TESTS FOR DISTINCTNESS, UNIFORMITY AND STABILITY prepared by experts from Hungary to be considered by the Technical Working Party for Agricultural Crops at its forty-seventh session, to be held in Naivasha, Kenya, from 2018-05-21 to 2018-05-25 Disclaimer: this document does not represent UPOV policies or guidance Alternative names:* Botanical name English French German Spanish Helianthus annuus L. Common Sunflower Soleil, Tournesol Sonnenblume Girasol The purpose of these guidelines (“Test Guidelines”) is to elaborate the principles contained in the General Introduction (document TG/1/3), and its associated TGP documents, into detailed practical guidance for the harmonized examination of distinctness, uniformity and stability (DUS) and, in particular, to identify appropriate characteristics for the examination of DUS and production of harmonized variety descriptions. ASSOCIATED DOCUMENTS These Test Guidelines should be read in conjunction with the General Introduction and its associated TGP documents. * These names were correct at the time of the introduction of these Test Guidelines but may be revised or updated. [Readers are advised to consult the UPOV Code, which can be found on the UPOV Website (www.upov.int), for the latest information.] TG/81/7(proj.1) Common Sunflower, 2018-04-05 2 TABLE OF CONTENTS PAGE 1. -

Effects of Root Pruning on Germinated Pecan Seedlings
PROPAGATION AND TISSUE CULTURE HORTSCIENCE 50(10):1549–1552. 2015. if pruning the roots shortly after germination stimulates lateral root formation. Similarly, little work has been attempted to consider Effects of Root Pruning on Germinated the effects of taproot pruning in young pecan seedlings back to different lengths on root Pecan Seedlings regeneration. The objective of this study 1 was to determine if root pruning at different Rui Zhang, Fang-Ren Peng , Pan Yan, Fan Cao, periods after seed germination affects pecan and Zhuang-Zhuang Liu seedling root and shoot growth and to evaluate College of Forestry, Nanjing Forestry University, 159 Longpan Road, different degrees of taproot pruning on root Nanjing 210037, China initiation. Dong-Liang Le Materials and Methods College of Forestry, Nanjing Forestry University, 159 Longpan Road, Nanjing 210037, China; and Nanjing Green Universe Pecan Science and The test was established at Lvzhou Pecan Research Station, Nanjing, China (lat. Technology Co. Ltd., 38 Muxuyuan Street, Nanjing 210007, China 32.05°N, long. 118.77°E). Open-pollinated Peng-Peng Tan seeds of the Chinese selection ‘Shaoxing’ were used as seed stock to produce seedlings. College of Forestry, Nanjing Forestry University, 159 Longpan Road, ‘Shaoxing’ had a small nut of very good Nanjing 210037, China quality with 50% percentage fill. The seeds averaged 30.4 mm in length, 20.9 mm in Additional index words. Carya illinoinensis, taproot, lateral root, shoot growth width, and 5.4 g in weight. Seeds were Abstract. Root systems of pecan trees are usually dominated by a single taproot with few collected on 7 Nov. -

Botany for Gardeners Offers a Clear Explanation of How Plants Grow
BotGar_Cover (5-8-2004) 11/8/04 11:18 AM Page 1 $19.95/ £14.99 GARDENING & HORTICULTURE/Reference Botany for Gardeners offers a clear explanation of how plants grow. • What happens inside a seed after it is planted? Botany for Gardeners Botany • How are plants structured? • How do plants adapt to their environment? • How is water transported from soil to leaves? • Why are minerals, air, and light important for healthy plant growth? • How do plants reproduce? The answers to these and other questions about complex plant processes, written in everyday language, allow gardeners and horticulturists to understand plants “from the plant’s point of view.” A bestseller since its debut in 1990, Botany for Gardeners has now been expanded and updated, and includes an appendix on plant taxonomy and a comprehensive index. Twodozen new photos and illustrations Botany for Gardeners make this new edition even more attractive than its predecessor. REVISED EDITION Brian Capon received a ph.d. in botany Brian Capon from the University of Chicago and was for thirty years professor of botany at California State University, Los Angeles. He is the author of Plant Survival: Adapting to a Hostile Brian World, also published by Timber Press. Author photo by Dan Terwilliger. Capon For details on other Timber Press books or to receive our catalog, please visit our Web site, www.timberpress.com. In the United States and Canada you may also reach us at 1-800-327-5680, and in the United Kingdom at [email protected]. ISBN 0-88192-655-8 ISBN 0-88192-655-8 90000 TIMBER PRESS 0 08819 26558 0 9 780881 926552 UPC EAN 001-033_Botany 11/8/04 11:20 AM Page 1 Botany for Gardeners 001-033_Botany 11/8/04 11:21 AM Page 2 001-033_Botany 11/8/04 11:21 AM Page 3 Botany for Gardeners Revised Edition Written and Illustrated by BRIAN CAPON TIMBER PRESS Portland * Cambridge 001-033_Botany 11/8/04 11:21 AM Page 4 Cover photographs by the author. -
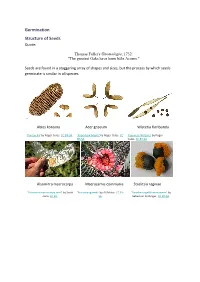
Germination Structure of Seeds Quote
Germination Structure of Seeds Quote: Thomas Fuller's Gnomologia, 1732: "The greatest Oaks have been little Acorns." Seeds are found in a staggering array of shapes and sizes, but the process by which seeds germinate is similar in all species. Abies koreana Acer griseum Wisteria floribunda 'Korean Fir' by Roger Culos. CC BY-SA. 'Paperbark Maple' by Roger Culos. CC 'Japanese Wisteria' by Roger BY-SA. Culos. CC BY-SA. Alsomitra macrocarpa Macrozamia communis Strelitzia reginae 'Alsomitra macrocarpa seed' by Scott 'BurrawangSeeds' by AYArktos. CC BY- 'Paradiesvogelblumensamen' by Zona. CC BY. SA. Sebastian Stabinger. CC BY-SA. Taraxicum officinale Stephanotis floribunda Phleum pratense 'Achane of Taraxacum sect. 'Stephanotis seed' by L. Marie"/Lenore 'Timoteegras vruchten Phleum Ruderalia' by Didier Edman, Sunnyvale, CA. CC BY. pratense' by Rasbak. CC BY-SA. Descouens. CC BY-SA. Dicotyledon seeds testa epicotyl plumule hypocotyl cotyledon radicle 'Aesculus hippocastanum seed section' by Boronian. CC BY. plumule epicotyl hypocotyl testa hilum radicle cotyledon micropyle endosperm Monocotyledon seeds endosperm epicotyl testa hypocotyl cotyledon radicle Parts of a seed Testa The seed coat. A protective layer which is tough and hard and it protects the seed from attack by insects, fungi and bacteria. Cotyledon Dicotyledons have 2 cotyledons Monocotyledons have 1 cotyledon A cotyledon is an embryonic leaf. It is the first leaf to appear when a seedling grows. They often contain reserves of food which the developing seedling can use to grow. Epicotyl The section of stem between the cotyledon(s) and the plumule. In a seedling it is the section of stem between the cotyledons and the first true leaves. -

Development and Cell Cycle Activity of the Root Apical Meristem in the Fern Ceratopteris Richardii
G C A T T A C G G C A T genes Article Development and Cell Cycle Activity of the Root Apical Meristem in the Fern Ceratopteris richardii Alejandro Aragón-Raygoza 1,2 , Alejandra Vasco 3, Ikram Blilou 4, Luis Herrera-Estrella 2,5 and Alfredo Cruz-Ramírez 1,* 1 Molecular and Developmental Complexity Group at Unidad de Genómica Avanzada, Laboratorio Nacional de Genómica para la Biodiversidad, Cinvestav Sede Irapuato, Km. 9.6 Libramiento Norte Carretera, Irapuato-León, Irapuato 36821, Guanajuato, Mexico; [email protected] 2 Metabolic Engineering Group, Unidad de Genómica Avanzada, Laboratorio Nacional de Genómica para la Biodiversidad, Cinvestav Sede Irapuato, Km. 9.6 Libramiento Norte Carretera, Irapuato-León, Irapuato 36821, Guanajuato, Mexico; [email protected] 3 Botanical Research Institute of Texas (BRIT), Fort Worth, TX 76107-3400, USA; [email protected] 4 Laboratory of Plant Cell and Developmental Biology, Division of Biological and Environmental Sciences and Engineering (BESE), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia; [email protected] 5 Institute of Genomics for Crop Abiotic Stress Tolerance, Department of Plant and Soil Science, Texas Tech University, Lubbock, TX 79409, USA * Correspondence: [email protected] Received: 27 October 2020; Accepted: 26 November 2020; Published: 4 December 2020 Abstract: Ferns are a representative clade in plant evolution although underestimated in the genomic era. Ceratopteris richardii is an emergent model for developmental processes in ferns, yet a complete scheme of the different growth stages is necessary. Here, we present a developmental analysis, at the tissue and cellular levels, of the first shoot-borne root of Ceratopteris. -

Auxins for Hardwood Cuttings: Effect of Root-Promoting Hormones
Auxins for Hardwood Cuttings effect of root-promoting hormones in propagating fruit trees by hardwood cuttings studied during past three seasons H. T. Hartmann Hardwood cuttings of five species of fruit trees, Marianna 2624 plum, Angers quince, Stockton Morello cherry, Mal- ling-Merton 793 apple, and Mission olive, were used in propagation tests to study the effects of various root-promot- ing hormones-auxins-applied under several different conditions. Marianna 2624 plum is a commonly used rootstock for a number of the stone fruit species; the 2624 selection is a seedling of the parent Marianna plum, presumably an open-pollinated cross of Prunus cerasifera and P. munsoniana. This rootstock is propagated commer- cially by hardwood cuttings, but in heavy soils considerable difficulty is often experienced in obtaining satisfac- tory rooting. Angers quince4ydonia oblong- has long been used as a dwarfing root- stock for certain of the pear varieties. It is commercially propagated by hard- wood cuttings. Stockton Morello cherry-Prunus cer- asus-is used to a considerable extent in California as a semidwarfing rootstock for the sweet cherry and is propagated commercially by suckers arising around the base of older trees. It would be de- sirable to be able to propagate this stock by cuttings. In all the tests conducted with this variety, however, not one hard- wood cutting was induced to root. Later studies have shown that it can be easily rooted under mist humidification by softwood cuttings taken from actively growing shoots if treated with indolebu- tyric acid. The Malling-Merton 793 apple-Ma- lus sylwstris-is a newly developed clonal apple rootstock from’ England which is usually propagated by some method of layering. -

Morphological Description of Plants: New Perspectives in Development and Evolution
® International Journal of Plant Developmental Biology ©2010 Global Science Books Morphological Description of Plants: New Perspectives in Development and Evolution 1* 2 Emilio Cervantes • Juana G . de Diego 1 IRNASA-CSIC. Apartado 257. 37080. Salamanca. Spain 2 Dept Bioquímica y Biología Molecular. Campus Miguel de Unamuno. Universidad de Salamanca. Spain Corresponding author : * [email protected] ABSTRACT The morphological description of plants has been fundamental in the history of botany and provided the keys for taxonomy. Nevertheless, in biology, a discipline governed by the interest in function and based on reductionist approaches, the analysis of forms has been relegated to second place. Plants contain organs and structures that resemble geometrical forms. Plant ontogeny may be seen as a sequence of growth processes including periods of continuous growth with modification that stop at crucial points often represented by structures of remarkable similarity to geometrical figures. Instead of the tradition in developmental studies giving more importance to animal models, we propose that the modular type of plant development may serve to remark conceptual aspects in that may be useful in studies with animals and contribute to original views of evolution. _____________________________________________________________________________________________________________ Keywords: concepts, description, geometry, structure INTRODUCTION: PLANTS OFFER NEW reflects a more general situation in Biology, a discipline APPROACHES TO DEVELOPMENT that has matured from the experimental protocols and views of biochemistry, where the predominant role of experimen- The study of development is today a major biological disci- tation has contributed to a decline in basic aspects that need pline. Although it was traditionally more focused in animal to be more descriptive, such as those related with mor- systems, increased emphasis in plant development may phology. -

Soybean Growth and Development & Management Information Fo Replant Decisions
MAGRCORE Metadata, citation and similar papers at core.ac.uk GOVSProvided by University of Minnesota Digital Conservancy MN 2500 AGFO-5701 MINNESOTA EXTENSION SERVICE I:,:, I UNIVERSITY OF MINNESOTA AGRICULTURE Soybean Growth and Development & Management Information fo UNIVf:R&TYoF MINNESOTA J DOCUMENTS ~ Replant Decisions JUL ·2s 1991 ,1 ·I shoot apex emerging decomposing radicle · - seed coat primary root L.L. Hardman J.L. Gunsolus Extension Agronomist-Crops Extension Agronomist-Weed Control Dept. of Agronomy & Dept. of Agronomy & Plant Genetics Plant Genetics Univ. of MN, St. Paul Univ. of MN, St. Paul SOYBEAN GROWTH AND DEVELOPMENT stored food is removed the cotyledons tum yellow and shrivel before dropping off the plant. Loss of one or more of the cotyledons before the food reserves are fully utilized can slow An understanding of how a soybean plant develops can help you early plant growth or result in death of the plant if photosynthetic make important management decisions. This section discusses leaf tissue is not formed quickly. various seed and plant parts, explains how the soybean plant develops utilizing standardized vegetative and reproductive stage descriptions which are used by the National Crop Insurance Services (NCIS), and describes some of the important factors which affect growth and development of the soybean plant. A soybean seed consists of a miniature plant attached to two nutrient storage reservoirs (cotyledons or seed halves) surrounded by a protective outer wrapper or seed coat called a testa (Figure 1). The cotyledons contain about 40% protein, 20% oil, and about 35% carbohydrate. These materials provide nutrients to the developing embryo during the germination process. -

Tree Seedling Availability, Planting, and Initial Care
F-5024 Tree Seedling Availability, Planting, and Initial Care William G. Ross Assistant Professor Oklahoma Cooperative Extension Fact Sheets Extension Forestry Specialistst are also available on our website at: http://www.osuextra.com Introduction This is the second in a series of three publications addressing the topic of forest stand establishment. If one is Bareroot not sure of what seedlings to plant or of what forest product to Most nurseries that sell seedlings produce some form of produce, it may be beneficial to read the first publication in this bareroot stock. Bareroot seedlings, as the name describes, is series, titled “Tree Planting Objectives and the Seedling a seedling with only the stem and the root supplied to the Selection Process.” landowner for planting. The major advantages of bareroot There are four points toconsider to identify management stock are the relative ease of production in the nurseries, the objectives. These are listed in brief form below. relative low cost to landowner, the relative ease of planting, and the ability to mechanize many of the operations in the 1. Identify the growing region where land is located. seedling production method. 2. Identify the soil types and moisture regimes located on the Shortcomings of bareroot seedlings include the vulner- land. ability of the scedling to the uncontrollable nursery environ- 3. Identify those objectives feasible and within the bounds of ment, exposure of the root system to harsh environmental the above environmental constraints. conditions after lifting, and the requirement of a large, sea- 4. Identify those tree species that, when managed correctly, sonal labor force to plant the seedlings. -

Life Cycles of Plants
Life Cycles of Plants Flowering plants produce flowers. The flowers later become fruit. Inside the fruit, we can find seeds. The fruit protect the seeds. Seeds can develop into new plants. The life cycle of flowering plants follows the three stages of seed-seedling-adult. Seed Seedling (Young plant) Stage 1: The plant begins Stage 2: The young plant that grows is known as a its life as a seed. With seedling. enough air, food, water and the right temperature, At first, the seedling obtains its food from the seed the seed will begin to leaves. germinate or grow. When the true leaves start to grow, the plant is ready to make its own food by the process of photosynthesis. The seed leaves will shrivel and fall off. Roots grow deep down into the ground to obtain water and mineral salts needed for the plant. The plant will grow towards the sunlight. The shoot grows upwards to obtain maximum sunlight. When the true leaves appear, the plant is ready to make its own food. Seed leaves provide the plant with food before the true leaves develop. Roots grow downwards to absorb water and mineral salts from the ground. They hold the plant firmly in the soil. Adult plant Stage 3: As the plant grows, it develops flowers which later become fruit. There are seeds inside the fruit. The seeds will fall to the ground and develop into new plants. The life cycle then repeats itself. Life cycle of a flowering plant Adapted: PSLE Science Partner A Complete Guide to L&U Block © Singapore Asia Publishers Pte Ltd.