International Union for the Protection of New Varieties of Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plant Physiology
PLANT PHYSIOLOGY Vince Ördög Created by XMLmind XSL-FO Converter. PLANT PHYSIOLOGY Vince Ördög Publication date 2011 Created by XMLmind XSL-FO Converter. Table of Contents Cover .................................................................................................................................................. v 1. Preface ............................................................................................................................................ 1 2. Water and nutrients in plant ............................................................................................................ 2 1. Water balance of plant .......................................................................................................... 2 1.1. Water potential ......................................................................................................... 3 1.2. Absorption by roots .................................................................................................. 6 1.3. Transport through the xylem .................................................................................... 8 1.4. Transpiration ............................................................................................................. 9 1.5. Plant water status .................................................................................................... 11 1.6. Influence of extreme water supply .......................................................................... 12 2. Nutrient supply of plant ..................................................................................................... -

What Are Soybeans?
candy, cakes, cheeses, peanut butter, animal feeds, candles, paint, body lotions, biodiesel, furniture soybeans USES: What are soybeans? Soybeans are small round seeds, each with a tiny hilum (small brown spot). They are made up of three basic parts. Each soybean has a seed coat (outside cover that protects the seed), VOCABULARY cotyledon (the first leaf or pair of leaves within the embryo that stores food), and the embryo (part of a seed that develops into Cultivar: a variety of plant that has been created or a new plant, including the stem, leaves and roots). Soybeans, selected intentionally and maintained through cultivation. like most legumes, perform nitrogen fixation. Modern soybean Embryo: part of a seed that develops into a new plant, cultivars generally reach a height of around 1 m (3.3 ft), and including the stem, leaves and roots. take 80–120 days from sowing to harvesting. Exports: products or items that the U.S. sells and sends to other countries. Exports include raw products like whole soybeans or processed products like soybean oil or Leaflets soybean meal. Fertilizer: any substance used to fertilize the soil, especially a commercial or chemical manure. Hilum: the scar on a seed marking the point of attachment to its seed vessel (the brown spot). Leaflets: sub-part of leaf blade. All but the first node of soybean plants produce leaves with three leaflets. Legume: plants that perform nitrogen fixation and whose fruit is a seed pod. Beans, peas, clover and alfalfa are all legumes. Nitrogen Fixation: the conversion of atmospheric nitrogen Leaf into a nitrogen compound by certain bacteria, such as Stem rhizobium in the root nodules of legumes. -

Effects of Root Pruning on Germinated Pecan Seedlings
PROPAGATION AND TISSUE CULTURE HORTSCIENCE 50(10):1549–1552. 2015. if pruning the roots shortly after germination stimulates lateral root formation. Similarly, little work has been attempted to consider Effects of Root Pruning on Germinated the effects of taproot pruning in young pecan seedlings back to different lengths on root Pecan Seedlings regeneration. The objective of this study 1 was to determine if root pruning at different Rui Zhang, Fang-Ren Peng , Pan Yan, Fan Cao, periods after seed germination affects pecan and Zhuang-Zhuang Liu seedling root and shoot growth and to evaluate College of Forestry, Nanjing Forestry University, 159 Longpan Road, different degrees of taproot pruning on root Nanjing 210037, China initiation. Dong-Liang Le Materials and Methods College of Forestry, Nanjing Forestry University, 159 Longpan Road, Nanjing 210037, China; and Nanjing Green Universe Pecan Science and The test was established at Lvzhou Pecan Research Station, Nanjing, China (lat. Technology Co. Ltd., 38 Muxuyuan Street, Nanjing 210007, China 32.05°N, long. 118.77°E). Open-pollinated Peng-Peng Tan seeds of the Chinese selection ‘Shaoxing’ were used as seed stock to produce seedlings. College of Forestry, Nanjing Forestry University, 159 Longpan Road, ‘Shaoxing’ had a small nut of very good Nanjing 210037, China quality with 50% percentage fill. The seeds averaged 30.4 mm in length, 20.9 mm in Additional index words. Carya illinoinensis, taproot, lateral root, shoot growth width, and 5.4 g in weight. Seeds were Abstract. Root systems of pecan trees are usually dominated by a single taproot with few collected on 7 Nov. -
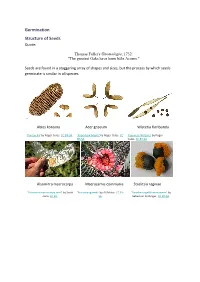
Germination Structure of Seeds Quote
Germination Structure of Seeds Quote: Thomas Fuller's Gnomologia, 1732: "The greatest Oaks have been little Acorns." Seeds are found in a staggering array of shapes and sizes, but the process by which seeds germinate is similar in all species. Abies koreana Acer griseum Wisteria floribunda 'Korean Fir' by Roger Culos. CC BY-SA. 'Paperbark Maple' by Roger Culos. CC 'Japanese Wisteria' by Roger BY-SA. Culos. CC BY-SA. Alsomitra macrocarpa Macrozamia communis Strelitzia reginae 'Alsomitra macrocarpa seed' by Scott 'BurrawangSeeds' by AYArktos. CC BY- 'Paradiesvogelblumensamen' by Zona. CC BY. SA. Sebastian Stabinger. CC BY-SA. Taraxicum officinale Stephanotis floribunda Phleum pratense 'Achane of Taraxacum sect. 'Stephanotis seed' by L. Marie"/Lenore 'Timoteegras vruchten Phleum Ruderalia' by Didier Edman, Sunnyvale, CA. CC BY. pratense' by Rasbak. CC BY-SA. Descouens. CC BY-SA. Dicotyledon seeds testa epicotyl plumule hypocotyl cotyledon radicle 'Aesculus hippocastanum seed section' by Boronian. CC BY. plumule epicotyl hypocotyl testa hilum radicle cotyledon micropyle endosperm Monocotyledon seeds endosperm epicotyl testa hypocotyl cotyledon radicle Parts of a seed Testa The seed coat. A protective layer which is tough and hard and it protects the seed from attack by insects, fungi and bacteria. Cotyledon Dicotyledons have 2 cotyledons Monocotyledons have 1 cotyledon A cotyledon is an embryonic leaf. It is the first leaf to appear when a seedling grows. They often contain reserves of food which the developing seedling can use to grow. Epicotyl The section of stem between the cotyledon(s) and the plumule. In a seedling it is the section of stem between the cotyledons and the first true leaves. -

Soybean Growth and Development & Management Information Fo Replant Decisions
MAGRCORE Metadata, citation and similar papers at core.ac.uk GOVSProvided by University of Minnesota Digital Conservancy MN 2500 AGFO-5701 MINNESOTA EXTENSION SERVICE I:,:, I UNIVERSITY OF MINNESOTA AGRICULTURE Soybean Growth and Development & Management Information fo UNIVf:R&TYoF MINNESOTA J DOCUMENTS ~ Replant Decisions JUL ·2s 1991 ,1 ·I shoot apex emerging decomposing radicle · - seed coat primary root L.L. Hardman J.L. Gunsolus Extension Agronomist-Crops Extension Agronomist-Weed Control Dept. of Agronomy & Dept. of Agronomy & Plant Genetics Plant Genetics Univ. of MN, St. Paul Univ. of MN, St. Paul SOYBEAN GROWTH AND DEVELOPMENT stored food is removed the cotyledons tum yellow and shrivel before dropping off the plant. Loss of one or more of the cotyledons before the food reserves are fully utilized can slow An understanding of how a soybean plant develops can help you early plant growth or result in death of the plant if photosynthetic make important management decisions. This section discusses leaf tissue is not formed quickly. various seed and plant parts, explains how the soybean plant develops utilizing standardized vegetative and reproductive stage descriptions which are used by the National Crop Insurance Services (NCIS), and describes some of the important factors which affect growth and development of the soybean plant. A soybean seed consists of a miniature plant attached to two nutrient storage reservoirs (cotyledons or seed halves) surrounded by a protective outer wrapper or seed coat called a testa (Figure 1). The cotyledons contain about 40% protein, 20% oil, and about 35% carbohydrate. These materials provide nutrients to the developing embryo during the germination process. -

Difference Between Radicle and Plumule Key Difference
Difference Between Radicle and Plumule www.differencebetween.com Key Difference - Radicle vs Plumule All seeds contain embryos. Seed embryo creates a new plant after germination. Therefore the embryo is protected inside the seed by a hard covering called testa. The seed begins to grow when the right conditions are found such as moisture, warmth, sunlight, nutrient-rich soil etc. There are two main components of a plant; shoot and root. Stem, leaves and roots develop from different parts of the seed embryo. The radicle is the first part that emerges from the seed during the germination through the micropyle (seed pore). It makes the roots of the new plant. Plumule emerges after the radicle and makes the stem of the new seedling. Cotyledons form the first leaves of the seedling. The key difference between radicle and plumule is that radicle is the root forming part of the seed embryo while plume is the stem forming part of the seed embryo. The cotyledons of the seed embryo hold the radicle and the plume. What is Radicle? The radicle is the embryonic root of the plant. It is a part of the seedling that comes first from the seed during the germination. It comes out from the seed through the micropyle. Radicle grows downwards into the soil. A root cap protects the tip of the radicle. It absorbs water and nutrients and supplies to the leaves for starting photosynthesis. Embryonic stem or the hypocotyl is found above the radicle. Figure 01: Radicle Radicle emerges from the seed as a short white structure. -

Early Soybean Growth and Development
EARLY SOYBEAN GROWTH AND DEVELOPMENT A soybean seed has two distinct parts: the cotyledons and the embryo. The two cotyledons are the main food storage structure, which supply food during emergence and for the seven to ten days after emergence through the V1 growth stage. The embryo itself is comprised of three parts. 1. The radicle is the first root and it’s the first part of the embryo to penetrate the seed coat. Lateral roots form from the radicle, and tiny root hairs then develop on the lateral roots. Root hairs are the main absorbing surface of the entire root system. A soybean’s root system branches and rebranches within the first four to five weeks after planting, with the bulk of root mass persisting in the top six inches of soil. 2. The hypocotyl is the stem below the cotyledon. It begins to elongate after the radicle and forms an arch, which is pushed upward. As the arch breaks the soil surface, it pulls the cotyledons and epicotyl up. The uppermost cells of the hypocotyl stop growing as cells on the underside continue to straighten the arch. This process lifts the cotyledons into an upright position. 3. The epicotyl is comprised of the small leaves, stem and the apical growing point. The epicotyl is protected between the cotyledons until after emergence. The loss of a cotyledon is not lethal to the seedling, but damage to the epicotyl is. The hypocotyl arch is a large structure that is relative to seed size, utilizing considerable seed energy to push through the soil. -

Growing a Sunflower from Seed
Growing a sunflower from seed First, make a pot for your sunflower seeds from newspaper. Fill the pot ¾ full with potting oil. Plant two seeds 1/4” deep in the center of your pot. When you get your pot home: Place your pot on a plastic plate or tray in a sunny window. Water your seeds very gently so you don’t wash the seeds or the soil out of the pot. Keep the soil damp but not wet. When the newspaper pot turns grey, you have watered enough. When the newspaper pot has turned white it is time to water again. Your plant will not need additional food (fertilizer) while it is growing in the pot. When your plants sprout, one will probably be bigger and stronger (dominant)- cut the weaker plant stem with scissors at the soil line. When the remaining plant is approximately 5”-6” tall and has two sets of true leaves, it is ready to be planted outside. The newspaper does not have to be removed when planting your plant as the newspaper will breakdown (decompose). You can plant your sunflower plant in a large container or in a prepared site like a garden. Be sure to plant your plant at the same soil line as it is growing in the pot. Enjoy! Germination of the Sunflower Seed After the seed is wetted and put in a warm dark place: First the fruit wall splits and radicle emerges Then the hypocotyl extends down and forms root hairs Plant development seed coat lateral cotyledons roots cotyledons . -

Variable Germination and Emergence in Soybean: Which Seeds Are Still Viable?
Variable Germination and Emergence in Soybean: Which Seeds Are Still Viable? Shawn P. Conley State Soybean and Wheat Extension Specialist Many of us, including myself, have planted under less than ideal soil conditions this spring. Often the ground was worked a little on the wet side leading to clods and variable seeding depths for our soybean crop. Early reports of variable and delayed emergence in conventional (more common) and notill soybean is raising replant and seed viability questions in several areas across the Midwest. If soybean was planted into dry soil and had not imbibed water (seed did not swell) then there is little to no concern for growers. Once a significant rainfall event occurs, the soybean will imbibe water, germinate, and should emerge normally. For yield estimates, we would assign the day it rained as the new planting date. The more difficult question to answer is “How viable is the soybean seed once imbibition and/or germination has begun?” The critical seed moisture content for soybean germination is 20%. A soybean seed that has imbibed water, has a split seed coat, or has an emerged radicle will continue to germinate and grow as normal once the seed is rehydrated if the seed (embryo) remains above 20% moisture (Senaratna and McKersie, 1983) (Image 1). If the moisture content within a soybean seed falls to10% due to dry conditions after germination has started, then a dramatic difference exists among the different seed germination stages. If the seed has imbibed water for 6 hours (seed is swollen, but the seed coat has not broken), then the seed is dehydrated to 10% moisture, germination is not affected (Image 2). -

Seed Viability, Germination, and Radicle Growth of Dwarf Mistletoe in California
PACIFIC SOUTHWEST Forest and Range FOREST SERVICE U. S. DEPARTMENT OF AGRICULTURE P.O. BOX 245, BERKELEY, CALIFORNIA 94701 Experiment Station U.S.D.A. FOREST SERVICE RESEARCH PAPER PSW- 59 /1970 Scharpf, Robert F. 1970. Seed viability, germination, and radicle growth of dwarf mistletoe in California. Berkeley, Calif. Pacific SW. Forest & Range Exp. Sta., 18 p., illus. (U.S.D.A. Forest Serv. Res. Paper PSW-59) Two species of dwarf mistletoe were studied: Arceuthobium abietinum (Engelm.) Hawksworth and Wiens and A. occidentale Engelm. Viability of fresh seeds was high and not significantly influenced by year of collection, where collected, or plant from which collected. Temperature affected viability most noticeably. It also significantly affected rate and percentage of seed germination. Longevity of radicles of germinated seeds was inversely proportional to temperatures above 5°C. Oxford: 176.1 Arceuthobium spp. (79):181.524/525-111.5. Retrieval Terms: Arceuthobium abietinum; Arceuthobium occidentale; seed viability; germination; temperature. Scharpf, Robert F. 1970. Seed viability, germination, and radicle growth of dwarf mistletoe in California. Berkeley, Calif. Pacific SW. Forest & Range Exp. Sta., 18 p., illus. (U.S.D.A. Forest Serv. Res. Paper PSW-59) Two species of dwarf mistletoe were studied: Arceuthobium abietinum (Engelm.) Hawksworth and Wiens and A. occidentale Engelm. Viability of fresh seeds was high and not significantly influenced by year of collection, where collected, or plant from which collected. Temperature affected viability most noticeably. It also significantly affected rate and percentage of seed germination. Longevity of radicles of germinated seeds was inversely proportional to temperatures above 5°C. Oxford: 176.1 Arceuthobium spp. -

R Ranunculus
R Ranunculus Maché™, Magic™ R A N U N C U L U S A S I A T I C U S GROWING ON Minimum Germination Rate: 80% High quality Ranunculus is best obtained with cool night temperatures and short Seed Product Form: Pelleted, raw day conditions. Low temperatures and high light levels will result in large flowers with an intense color. FLOWERING Transplant Ready: 8 – 10 weeks from sow in a ‘288’ tray. Time frame when plants are receptive to flower initiation: 10 – 12 leaves are Finish Bulking/Flower Initiation: Optimum conditions during the vegetative present. period, beginning at transplant, needed for the root to reach the edge of the Flowering Type: Obligate long-day plant – long days required for initiation. container AND to make the plant receptive to flower initiation. Specific Flowering Mechanism: Days greater than 13.5 hours with high Media: Select a porous media that drains well. This is important during the cool irradiance (15 – 20 mols) will induce and enhance flowering. After initiation and season when temperatures and light levels are low, and media is slow to dry. while buds are immature, provide short day to enhance flower size and uniformity. • pH: 5.8 – 6 • EC: 1.25 – 1.75 PLUG CULTURE Light: 4,500 – 5,000 foot candles (45,000 – 50,000 lux or 16 – 18 mols of light). Germination: Optimum conditions for seedling development that begins the day Ranunculus initiates the highest flower count and the best growth under the the crop is sown until cotyledon expansion. Expect radicle emergence in 14 – 21 natural days for spring production. -

Induced Radicle Rooting : an Alternative Technique for Rapid and Mass Screening of Rice Genetic Accessions for Deep Root System
Induced Radicle Rooting : An alternative Technique for Rapid and Mass screening of Rice Genetic Accessions for Deep Root system Gomathinayagam Pichiah ( [email protected] ) Wollega University https://orcid.org/0000-0001-8468-8698 Beka Biri Wollega University Original article Keywords: Deep root accession, Drought screening, Induced radicle root , IRR technique Posted Date: October 6th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-76659/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/19 Abstract Drought is a recurrent phenomenon in the fragile crop production environments. Drought resistant crop varieties strike roots deeper into the soil to withstand drought spells during the crop growth period better than the shallow rooted varieties. Thus the deeper they strike roots under stress, the better their survival. Plant breeding for less than ideal condition is very dicult especially for breeding resistance to drought. The existing screening techniques are based on lateral root or brous root development for monocots like rice or barley .This paper explains a new innovative approach called Induced Radicle Rooting (IRR) technique which is a novel technique in which radicle root is allowed to grow by suppressing the growth of the lateral roots. The induction of radicle rooting is achieved in three steps viz.(i) by allowing to touch the radicle of the germinated seed on the water level keeping other parts above. (ii) by reducing the water level of the well periodically then and there when the radicle starts growing (iii) by allowing the growth of the radicle down deeper in the capillary tube in such a way by arresting crown and lateral roots.