Groundwater Quality Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
KERALA STATE ELECTRICITY BOAIID LIMIT[T)
I KERALA STATE ELECTRICITY BOAIID LIMIT[t) . (Incorporoted under the Contponies Act, 1956) Reg.Office : Vyciyuthi l3havanam, Pattom' Thiruvanantlta;luranr - 695 004 i CI N : U40 1 0 0 K t .20 r1^s}c027 424 Wcbs itc: rvlv',v.ksclt. n. Phone: +91,4772574576,24468tt5,9446008ti84 1]-rnilil: trtrl<-st:[r(o)l<stlbnet.coni. citl<seb@]<seb.in AESTRACT Downstream works for the upcoming 22OkV Substation, Thuravoor - Sanction accorded - Orders issued. Corporate Office (SBU-Tl B.O.(DB)No.3g2l\O2O/D(T,SO&S)/T1lThuravui Down srream works/2020-21/Thiruvananthapuram dated 05.06.2020 Read: 1. Letter No.CE(TS)EE:^/AEEIV/t9-2O/I773 dated 30.10.2019 of the chief Engineer (Trans. south) 2. Lener No.CE(TS)EEt/AEEIV/2O-2t/1O6 dated 28.O4.2O2O of the Chief Engineer (Trans. South) 3. Note No.D(LSO&S)lTl/Thuravur Down Stream Works/19-20 (Agenda ltem No.46l5/2O) 4. Note No.D(I,SO&S)/T1/Thuravur Down Stream Works/19-21lt95 of the chairman & Managing Director. 5. Proceedings of the 53'd meeting of the Board of Directors held on 25.05.2020 (Agenda i 19-05/2020) ORDER KSEBL is constructing a 22OkV Substation at Thuravoor, Cherthala Taluk, under TransGrid project with 2 Nos. of 2OOMVA, zzlltt}kV Transformers and 8Nos. of L10kV outgoing feeders. The Chief Engineer (Transmission South) as per letter read as 1n above submitted a proposal amounting to Rs.77.72Cr for the downstream evacuation works of the 220kV Substation, Thuravoor. The downstream line work includes construction of 37km of 110kV DC feeders by upgrading the existing 66KV SC Vaikom-Cherthala feeders and there after interlinking with the existing 110KV DC feeders to facilitate as outgoing 110kV feeders from the 220kV Substation, Thuravur. -

Payment Locations - Muthoot
Payment Locations - Muthoot District Region Br.Code Branch Name Branch Address Branch Town Name Postel Code Branch Contact Number Royale Arcade Building, Kochalummoodu, ALLEPPEY KOZHENCHERY 4365 Kochalummoodu Mavelikkara 690570 +91-479-2358277 Kallimel P.O, Mavelikkara, Alappuzha District S. Devi building, kizhakkenada, puliyoor p.o, ALLEPPEY THIRUVALLA 4180 PULIYOOR chenganur, alappuzha dist, pin – 689510, CHENGANUR 689510 0479-2464433 kerala Kizhakkethalekal Building, Opp.Malankkara CHENGANNUR - ALLEPPEY THIRUVALLA 3777 Catholic Church, Mc Road,Chengannur, CHENGANNUR - HOSPITAL ROAD 689121 0479-2457077 HOSPITAL ROAD Alleppey Dist, Pin Code - 689121 Muthoot Finance Ltd, Akeril Puthenparambil ALLEPPEY THIRUVALLA 2672 MELPADAM MELPADAM 689627 479-2318545 Building ;Melpadam;Pincode- 689627 Kochumadam Building,Near Ksrtc Bus Stand, ALLEPPEY THIRUVALLA 2219 MAVELIKARA KSRTC MAVELIKARA KSRTC 689101 0469-2342656 Mavelikara-6890101 Thattarethu Buldg,Karakkad P.O,Chengannur, ALLEPPEY THIRUVALLA 1837 KARAKKAD KARAKKAD 689504 0479-2422687 Pin-689504 Kalluvilayil Bulg, Ennakkad P.O Alleppy,Pin- ALLEPPEY THIRUVALLA 1481 ENNAKKAD ENNAKKAD 689624 0479-2466886 689624 Himagiri Complex,Kallumala,Thekke Junction, ALLEPPEY THIRUVALLA 1228 KALLUMALA KALLUMALA 690101 0479-2344449 Mavelikkara-690101 CHERUKOLE Anugraha Complex, Near Subhananda ALLEPPEY THIRUVALLA 846 CHERUKOLE MAVELIKARA 690104 04793295897 MAVELIKARA Ashramam, Cherukole,Mavelikara, 690104 Oondamparampil O V Chacko Memorial ALLEPPEY THIRUVALLA 668 THIRUVANVANDOOR THIRUVANVANDOOR 689109 0479-2429349 -

Scheduled Caste Sub Plan (Scsp) 2014-15
Government of Kerala SCHEDULED CASTE SUB PLAN (SCSP) 2014-15 M iiF P A DC D14980 Directorate of Scheduled Caste Development Department Thiruvananthapuram April 2014 Planng^ , noD- documentation CONTENTS Page No; 1 Preface 3 2 Introduction 4 3 Budget Estimates 2014-15 5 4 Schemes of Scheduled Caste Development Department 10 5 Schemes implementing through Public Works Department 17 6 Schemes implementing through Local Bodies 18 . 7 Schemes implementing through Rural Development 19 Department 8 Special Central Assistance to Scheduled C ^te Sub Plan 20 9 100% Centrally Sponsored Schemes 21 10 50% Centrally Sponsored Schemes 24 11 Budget Speech 2014-15 26 12 Governor’s Address 2014-15 27 13 SCP Allocation to Local Bodies - District-wise 28 14 Thiruvananthapuram 29 15 Kollam 31 16 Pathanamthitta 33 17 Alappuzha 35 18 Kottayam 37 19 Idukki 39 20 Emakulam 41 21 Thrissur 44 22 Palakkad 47 23 Malappuram 50 24 Kozhikode 53 25 Wayanad 55 24 Kaimur 56 25 Kasaragod 58 26 Scheduled Caste Development Directorate 60 27 District SC development Offices 61 PREFACE The Planning Commission had approved the State Plan of Kerala for an outlay of Rs. 20,000.00 Crore for the year 2014-15. From the total State Plan, an outlay of Rs 1962.00 Crore has been earmarked for Scheduled Caste Sub Plan (SCSP), which is in proportion to the percentage of Scheduled Castes to the total population of the State. As we all know, the Scheduled Caste Sub Plan (SCSP) is aimed at (a) Economic development through beneficiary oriented programs for raising their income and creating assets; (b) Schemes for infrastructure development through provision of drinking water supply, link roads, house-sites, housing etc. -
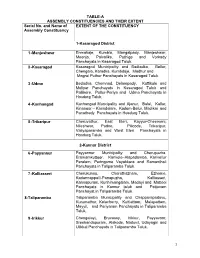
List of Lacs with Local Body Segments (PDF
TABLE-A ASSEMBLY CONSTITUENCIES AND THEIR EXTENT Serial No. and Name of EXTENT OF THE CONSTITUENCY Assembly Constituency 1-Kasaragod District 1 -Manjeshwar Enmakaje, Kumbla, Mangalpady, Manjeshwar, Meenja, Paivalike, Puthige and Vorkady Panchayats in Kasaragod Taluk. 2 -Kasaragod Kasaragod Municipality and Badiadka, Bellur, Chengala, Karadka, Kumbdaje, Madhur and Mogral Puthur Panchayats in Kasaragod Taluk. 3 -Udma Bedadka, Chemnad, Delampady, Kuttikole and Muliyar Panchayats in Kasaragod Taluk and Pallikere, Pullur-Periya and Udma Panchayats in Hosdurg Taluk. 4 -Kanhangad Kanhangad Muncipality and Ajanur, Balal, Kallar, Kinanoor – Karindalam, Kodom-Belur, Madikai and Panathady Panchayats in Hosdurg Taluk. 5 -Trikaripur Cheruvathur, East Eleri, Kayyur-Cheemeni, Nileshwar, Padne, Pilicode, Trikaripur, Valiyaparamba and West Eleri Panchayats in Hosdurg Taluk. 2-Kannur District 6 -Payyannur Payyannur Municipality and Cherupuzha, Eramamkuttoor, Kankole–Alapadamba, Karivellur Peralam, Peringome Vayakkara and Ramanthali Panchayats in Taliparamba Taluk. 7 -Kalliasseri Cherukunnu, Cheruthazham, Ezhome, Kadannappalli-Panapuzha, Kalliasseri, Kannapuram, Kunhimangalam, Madayi and Mattool Panchayats in Kannur taluk and Pattuvam Panchayat in Taliparamba Taluk. 8-Taliparamba Taliparamba Municipality and Chapparapadavu, Kurumathur, Kolacherry, Kuttiattoor, Malapattam, Mayyil, and Pariyaram Panchayats in Taliparamba Taluk. 9 -Irikkur Chengalayi, Eruvassy, Irikkur, Payyavoor, Sreekandapuram, Alakode, Naduvil, Udayagiri and Ulikkal Panchayats in Taliparamba -
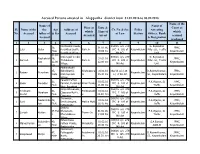
Accused Persons Arrested in Alappuzha District from 31.01.2016 to 06.02.2016 Name of the Name of Name of Place at Date & Court at Sl
Accused Persons arrested in Alappuzha district from 31.01.2016 to 06.02.2016 Name of the Name of Name of Place at Date & Court at Sl. Name of the the Age Address of Cr. No & Sec Police Arresting which Time of which No. Accused father of & Sex Accused of Law Station Officer, Rank Arrested Arrest accused Accused & Designation produced 1 2 3 4 5 6 7 8 9 10 11 Koottunkal Veedu, 358/16 u/s 279 G. Rajendran 38, 31.01.16, JFMC, 1 Lalu Balan Keerikkad South, Park Jn IPC & 185 of Kayamkulam Pillai. SI , Traffic Male 10.00 hrs Kayamkulam Keerikkad Village MV ACT Unit 31.01. Krishnajali Veedu, 359/16 u/s 279 G. Rajendran Radhakrish 16, 31.01.16 JFMC, 2 Sareesh Thekkakara Park Jn IPC & 185 of Kayamkulam Pillai. SI , Traffic nan 12.00 12.00 hrs Kayamkulam Village MV Act Unit hrs Muthirakuzhi 35, Kizhakkathil, Krishnapura 31.01.16 362/16 u/s 118 ( D.Rajeeshkumar, JFMC, 3 Rajeev Purushan Kayamkulam male Krishnapuam m 16.30 hrs a ) of KA Act SI , Kayamkulam Kayamkulam Village Paradiil Puthen 363/16 u/s 279 25, 01.02.16 P.K.Vijayan, SI JFMC, 4 Sagar Devadas Parmbil, Purakkad Kinar Mukku IPC & 185 of Kayamkulam Male 11.00 hrs Traffic Kayamkulam Muri, Purakkad MV Act Simjo Bhavanam, 364/16 u/s 279 Ferdinant 31, Pullikkanakk 01.02.16 P.K.Vijayan, SI JFMC, 5 Abraham Clappana North, IPC 7 185 of Kayamkulam Peeter Male u 12.30 hrs Traffic Kayamkulam Clappana MV Act Thriveniyil, 365/16 u/s 279 Neelambha 43, 01.02.16 P.K.Vijayan, SI JFMC, 6 Sunil Devikulangara, Mahila Mukk IPC & 185 of Kayamkulam ran Male 12.45 hrs Traffic Kayamkulam Puthuppally MV Act Shubha -

ANNUAL REPORT 2019-2020 (To Be Placed Before the Kerala Legislative Assembly, As Per Section 105 of Electricity Act, 2003) 2 KSERC Annual Report 2019-20 CONTENTS
KERALA STATE ELECTRICITY REGULATORY COMMISSION ANNUAL REPORT Kerala State Electricity Regulatory Commission 2019-20 KPFC Bhavanam, C.V. Raman Pillai Road, Vellayambalam, Thiruvananthapuram 695 010 Phone: 0471-2735544 | Fax: 0471-2735599 | Email: [email protected] | Website : www.erckerala.org St. Joseph’s Press, Tvm KERALA STATE ELECTRICITY REGULATORY COMMISSION ANNUAL REPORT 2019-2020 (To be placed before the Kerala Legislative Assembly, as per Section 105 of Electricity Act, 2003) 2 KSERC Annual Report 2019-20 CONTENTS 1. Introduction 2. The Commission 3. Functions of the Commission 4. Chairman & Members of the Commission 5. Office of the Commission 6. State Advisory Committee 7. State Co-ordination Forum 8. Licensees 9. Proceedings of the Commission 10. Regulations issued by the Commission 11. Abstract of disposal of petitions 12. Orders issued by the Commission 13. Application of Information Technology 14. Associations with other bodies 15. Consumer Grievance Redressal Forum (CGRF) 16. Electricity Ombudsman 17. Compliance of the Electricity Act, 2003, and the Rules and Regulations made thereunder. 18. Engagement of Auditors 19. Implementation of Right to Information Act 20. Legal Matters 21. Finance and Account 22. COVID – 19 Pandemic Annexure I- List of proceedings/hearing held by the Commission during 2019-20 Annexure II – List of inspections conducted by Junior Consultants during 2019-20 Annexure III- Annual Accounts 2019-20 Annexure IV- Separate Audit report of the Comptroller and Audit General of India on the Accounts for the year 2018-19 KSERC Annual Report 2019-20 3 4 KSERC Annual Report 2019-20 1. INTRODUCTION The Kerala State Electricity Regulatory Commission, established in the year 2002, entered into its 18th year of service in 2019-20. -

Accused Persons Arrested in Alappuzha District from 10.05.2020To16.05.2020
Accused Persons arrested in Alappuzha district from 10.05.2020to16.05.2020 Name of Name of Name of the Place at Date & Arresting the Court Name of the Age & Cr. No & Police Sl. No. father of Address of Accused which Time of Officer, at which Accused Sex Sec of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 Cr No-505 /2020 U/S188, 269 IPC & Sec. 4(2)(a) r/w 5 1 of Kerala Epidemic Kudappurakizakka 16-05- Diseases Aneeshkum 37 thil,Kozhuvallor Kollakadav 2020 Ordinance BAILED ar Chellapan Male Po,Mulakazha u 21:12 2020 VENMANI Pradeep S BY POLICE Veliyil Nikarth Trichattukulam 16-05- Cr No-824 2 63 P.o Panavally Trichattuku 2020 /2020 U/S15 POOCHAK Mithran BAILED Mohanan Kesavan Male P/W-4 lam 20:24 of KG Act AL K.M BY POLICE Kuzhikkattuchira Trichattukulam 16-05- Cr No-824 3 Pushpanga 39 P.O Panavally Trichattuku 2020 /2020 U/S15 POOCHAK Mithran BAILED Anilkumar dhan Male P/W-4 lam 20:24 of KG Act AL K.M BY POLICE Vattachira Trichattukulam 16-05- Cr No-824 4 38 P.O Panavally Trichattuku 2020 /2020 U/S15 POOCHAK Mithran BAILED Sajeev Raghavan Male P/W-4 lam 20:24 of KG Act AL K.M BY POLICE Kuzhiparambil Trichattukulam 16-05- Cr No-824 5 56 P.o Panavally Trichattuku 2020 /2020 U/S15 POOCHAK Mithran BAILED Vijayan Kumaran Male P/W-4 lam 20:24 of KG Act AL K.M BY POLICE thai parambu, Cr No-573 Bappu vaidyar Jn, 16-05- /2020 6 26 Canal ward, zakkariya 2020 U/S279,194( ALAPPUZ KABEER BAILED Azeem Koyamon Male Alappuzha bazar 20:00 D) of MV Act HA SOUTH C E BY POLICE Cr No-543 /2020 U/S188 Ipc 118(e) -

Panchayat/Municipality/Corp Oration
PMFBY List of Panchayats/Municipalities/Corporations proposed to be notified for Rabi II Plantain 2018-19 Season Insurance Unit Sl State District Taluka Block (Panchayat/Municipality/Corp Villages No oration) 1 Kerala Thiruvananthapuram Athiyannoor Kanjiramkulam All Villages in the Notified Panchayats 2 Kerala Thiruvananthapuram Athiyannoor Karimkulam All Villages in the Notified Panchayats 3 Kerala Thiruvananthapuram Athiyannoor Athiyanoor All Villages in the Notified Panchayats 4 Kerala Thiruvananthapuram Athiyannoor Kottukal All Villages in the Notified Panchayats 5 Kerala Thiruvananthapuram Athiyannoor Venganoor All Villages in the Notified Panchayats 6 Kerala Thiruvananthapuram Chirayinkeezhu Kizhuvilam All Villages in the Notified Panchayats 7 Kerala Thiruvananthapuram Chirayinkeezhu Mudakkal All Villages in the Notified Panchayats 8 Kerala Thiruvananthapuram Chirayinkeezhu Anjuthengu All Villages in the Notified Panchayats 9 Kerala Thiruvananthapuram Chirayinkeezhu Chirayinkeezhu All Villages in the Notified Panchayats 10 Kerala Thiruvananthapuram Chirayinkeezhu Kadakkavoor All Villages in the Notified Panchayats 11 Kerala Thiruvananthapuram Chirayinkeezhu Vakkom All Villages in the Notified Panchayats 12 Kerala Thiruvananthapuram Kilimanoor Madavoor All Villages in the Notified Panchayats 13 Kerala Thiruvananthapuram Kilimanoor Pallickal All Villages in the Notified Panchayats 14 Kerala Thiruvananthapuram Kilimanoor Kilimanoor All Villages in the Notified Panchayats 15 Kerala Thiruvananthapuram Kilimanoor Nagaroor All Villages -

Industrial Profile- Alappuzha District 2016-2017
Government of India Ministry of MSME Brief Industrial Profile of Alappuzha District 2016-17 Carried out by MSME – Development Institute, Thrissur Ayyanthole P.O., Kanjani Road, Kerala – 680003 Email: [email protected] Website: www.msmsedithrissur.gov.in Phone: 0487-2360536, Fax: 0487-2360216 FOREWORD MSME-DI, Thrissur, as part of its activities has prepared the District Industrial Profile of Alappuzha District during 2016-17. The objective to prepare the Industrial Profile of Alappuzha is to make the information readily available in respect of general characteristics of the district, administrative set up, industrial scenario, potential for new MSMEs, details of existing clusters and general issues raised by industry associations etc. to the existing as well as prospective entrepreneurs. It is expected that this Industrial Profile will be useful for Governmental & Non-Governmental organizations, Promotional & Developmental Agencies engaged in promotion & development of MSME sector in Alappuzha District and also for academicians / research scholars. I take this opportunity to express my gratitude to District Industries Centre, Alappuzha, officials of State Planning Board, Economic and Statistics Department, Kerala, Department of Mines and Geology and heads of other departments for extending their co-operation in preparing this Industrial Profile. I also place on records my appreciation to Smt. Kathreenamma Sebastian, Deputy Director (E.I.), and Shri. G. Balagopal, Asst Director (Stat) of this institute for their hard work in bringing out the report in this form. Thrissur 31.03.2017 CONTENTS S. No. Topic Page No. 1. General Characteristics of the District 3 1.1 Location & Geographical Area 3 1.2 Topography 3 1.3 Availability of Minerals. -

KELTRON CONTROLS, AROOR Page 1 of 2
KELTRON CONTROLS, AROOR KERALA STATE ELECTRONICS DEVELOPMENT CORPORATION LIMITED (A Government of Kerala Undertaking) KELTRON CONTROLS DIVISION, AROOR, ALAPPUZHA Dist, Kerala -688534 Tel: +91 478-2872323 to 26 , 0478- 2830700 Fax: +91 478-2872322 e-mail : [email protected] [email protected] Website : www.keltron.org NOTICE INVITING TENDER (NIT) (e-Procurement) Tender No : KSEDC/KCA/PPD/002/2017-18 dated 11.01.2018 1. Kerala State Electronics Development Corporation Limited, KELTRON CONTROLS DIVISION, AROOR, ALAPPUZHA Dist., Kerala PIN 688534, (Herein as referred to as KELTRON) invites e-tenders from Original Equipment Manufacturer or its Authorized Channel partner/Distributor/Dealer for entering into a Rate Contract for a period of 6 months from the date of finalization of rate Contract for supply of Scooter with Additional Retrofit for Physically Challenged Persons, 4 stroke and Electric start as per the Schedule of Requirements given below and as per BOQ, meeting the Technical Specifications, subject to General & Special Terms & Conditions as per the Tender Document furnished with this tender. 2. Items to be delivered to our various sites at various Panchayaths / Municipalities / Corporations mainly of Trichur, Ernakulam, Kottayam, Idukki & Alappuzha districts and also for other districts in Kerala State. Schedule of Requirement Sl. No. Item Description Quantity ( Nos) 1 Type 1: Scooter with 4stroke and Electric start (Capacity: 100cc to 105cc) (specifications as per annexure 1 of Tender Document) 1a Additional Retrofit for Type 1: - 200(Approx.) Two side wheels having shock absorbers (specifications as per annexure 1 of Tender Document) 2 Type 2: Scooter with 4stroke and Electric start (Capacity: 105cc to 110cc) (specifications as per annexure 1 of Tender Document) 2a Additional Retrofit for Type 2: - 300 (Approx.) Two side wheels having shock absorbers (specifications as per annexure 1 of Tender Document) Page 1 of 2 KELTRON CONTROLS, AROOR 3. -

Details of the Dealership of Hpcl to Be Uploaded in the Portal South Zone State:Kerala Sr
Details in subsequent pages are as on 01/04/12 For information only. In case of any discrepancy, the official records prevail. DETAILS OF THE DEALERSHIP OF HPCL TO BE UPLOADED IN THE PORTAL SOUTH ZONE STATE:KERALA SR. No. Regional Office State Name of dealership Dealership address (incl. location, Dist, State, PIN) Name(s) of Proprietor/Partner(s) Outlet Telephone No. HPCL DEALERS, 13/770, NEAR NOORANAD JN., KP ROAD, 1 Cochin Kerala A S FUELS, NOORNAD NOORNADU, ALAPPUZHA DISTRICT, PIN:690504, KERALA MURALIDHARAN NAIR 9388867230 STATE. HPCL DEALERS, MC ROAD, VENJARAMUD, TRIVANDRUM 2 Cochin Kerala A.K. Jameela Begum A.K. Jameela Begum, Sheeja Shafi 9495154958 DISTRICT, PIN:695607, KERALA STATE. HPCL DEALERS, Chakkaraparambu, Ernakulam NH By Pass, 3 Cochin Kerala A.M. Sadick, NH Byepass Kanayannur, Ernakulam DISTRICT, PIN:682032, KERALA A.M. Sadick 9895290824 STATE. HPCL DEALERS, WARD 4/614 B, OPP: TASTE BUDS HOTEL, 4 Cochin Kerala A.N. Raman Pillai & Sons,Koothattukulam KOOTHATUKULAM JUNCTION, MC ROAD, KOOTHATUKULAM , R. Suresh kumar ERNAKULAM DISTRICT, PIN:686662, KERALA STATE. HPCL DEALERS, 378 WARD 8, NEAR NAINAR MOSQUE, NH 5 Cochin Kerala Al Ameen Corporation, Kanjirapally 220, KANJIRAPALLY, KOTTAYAM DISTRICT, PIN:686507, M.M. Syed Mohammed 9447316820 KERALA STATE. HPCL DEALERS, 15/393, NH-208, KOTTARAKARA, KOLLAM 6 Cochin Kerala Aleyamma Mathew, Kadappakada Prasad Mathew 9605006835 DISTRICT, PIN:691506, KERALA STATE. HPCL DEALERS, NEAR SASTRI JN, QS RD, KOLLAM, KOLLAM 7 Cochin Kerala Aleyamma Mathew, Kottarakkara Mathew Idiculla 9895974254 DISTRICT, PIN:691001, KERALA STATE. HPCL DEALERS, "5/1, NEAR Paravur Kavala, Paravur Kavala on 8 Cochin Kerala Alwaye Business Corporation, Alwaye NH-47, Alwaye, Ernakulam DISTRICT, PIN:683101, KERALA Smt. -

Koluthara Exports Ltd, Keltron Road, Aroor, Alappuzha, Kerala
KOLUTHARA EXPORTS LTD, KELTRON ROAD, AROOR, ALAPPUZHA, KERALA ANNUAL REPORT FOR THE PERIOD 2019 - 2020 CONTENTS PAGES 1. BOARD OF DIRECTORS 1 2. NOTICE 2 - 10 3. DIRECTORS' REPORT 11 - 21 4. INDEPENDENT AUDITORS REPORT 22 - 35 5. BALANCE SHEET 36 6. PROFIT AND LOSS ACCOUNT 37 7. CASH FLOW STATEMENT 38 8. NOTE NO.1 SIGNIFICANT ACCOUNTING POLICIES 39 - 41 9. NOTE 10 DEPRECIATION STATEMENT 42 10. NOTES 2 TO 35 43 - 54 31 st Annual Report 2020 KOLUTHARA EXPORTS LTD, KELTRON ROAD, AROOR, ALAPPUZHA, KERALA BOARD OF DIRECTORS ANTONY VARGHESE KOLUTHARA Managing Director GRACE ANTONY KOLUTHARA Director P.A VARGHESE Director JOSEPH JOSEPH Director K.J. JOSEPH Director REGISTERED OFFICE & FACTORY VII/ 45 , Keltron Road, Aroor - 688 534 , Alappuzha District, Kerala. CIN - U51909KL1989PLC005486 BANKERS Federal Bank Ltd Aroor, Alappuzha Dist. - 688 534 AUDITORS M/s. George, John and Prabhu, Chartered Accountants, Cochin - 682 016 . Registrar & Transfer Agent BTS Consultancy Services Pvt. Ltd., 1st Floor, M S Complex, Plot No.8, Sastri Nagar, Near Rettery / Beh RTO North, Kolathur, Chennai – 600099 31 st Annual Report 2020 KOLUTHARA EXPORTS LTD, KELTRON ROAD, AROOR, ALAPPUZHA, KERALA NOTICE Notice is hereby given that the 31 st Annual General Meeting of the Members of Koluthara Exports Ltd will be held on Tuesday, 22 nd December, 2020, at 11.30 a.m., through Video Conferencing (VC) / Other Audio Visual Means (OAVM) in accordance with the applicable provisions of the Companies Act, 2013 read with MCA General Circular No. 20/2020, 14/2020 and 17/2020 dated 5 th May, 2020, 8 th April, 2020 and 13 th April, 2020 to transact the following business.