INTERNATIONAL CONFERENCE in ORGANIC SYNTHESIS 2016 ,"Frm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MELAKA Business Name Outlet Address State NASI ARAB 115 NO 76 JALAN LINGKARAN MITC, KOMPLEKS PERNIAGAAN MITC PERDANA, 75450 AYER KEROH, MELAKA
MELAKA Business Name Outlet Address State NASI ARAB 115 NO 76 JALAN LINGKARAN MITC, KOMPLEKS PERNIAGAAN MITC PERDANA, 75450 AYER KEROH, MELAKA. MELAKA MERLIMAU BURGER STATION NO JA 1471 , JLN JASIN, TMN MUHIBBAH , MER 77300 MELAKA BUSUINA ENTERPRISE GERAI NO 10,TMN MERLIMAU BARU,MER77300 MELAKA INSPIRASI JUTA JA 8008,KG SERKAM PANTAI, MER 77300 MELAKA CUTE GF HAIR SALOON JC118 , JLN BMU 2, BDR BARU MERLIMAU, MER 77300 MELAKA RESTORAN KARI KAMBING 41 HARI BT 20, JLN TAMBAK MERAH , SG RAMBAI 77400 MELAKA PERNIAGAAN MUSLIM SU 931 & 932, RUMAH KEDAI SETINGKAT, BANDAR BARU MASJID TANAH 78300 MELAKA RIZ JAYA BATU 33 3/4, KG SG JERNEH , LUBOK CHINA 78100 MELAKA KAFE SERI BALKIS PT 925 PT 2704, JLN BANDAR BARU 6, TAMAN BANDAR BARU, 78300 MASJID TANAH, MELAKA MELAKA PERUSAHAAN MAKANAN & PENGAWETAN ISTIMEWA KG AIR HITAM PANTAI, 78300 MASJID TANAH, MELAKA MELAKA CIK CHINTA SU877, PUSAT PERNIAGAAN BANDAR BARU MASJID TANAH, 78300 MASJID TANAH, MELAKA MELAKA BERKAT SHAYZ ENT NO 6972 BT 20 1/4, KG AIR LIMAU, 78300 MASJID TANAH, MELAKA MELAKA MHA STAR RESOURCES SU 506 JLN MAWAR 3, TMN SG BARU, 78300 MASJID TANAH, MELAKA MELAKA RESTOREN MADINA 254, JALAN MELAKA RAYA 3, TMN MELAKA RAYA, 75000 MELAKA ALONG FIRDAUS CAFÉ ALONG FIRDAUS CAFÉ, GERAI NO 8, DEWAN BENTARA, UITM CAMPUS, MELAKA 78000 MELAKA BOLLYWOOD MAJU ENTERPRISE NO 1, JLN KRISTAL MERAH 2, TAMAN LIMBONGAN JAYA 75200 MELAKA RICHIAMO COFFEE STUDENT BUSINESS CENTRE, UITM ALOR GAJAH 78000 MELAKA EV OPTICAL AG3743, JLN BESAR, ALOR GAJAH 78000 MELAKA AIDAMANSHAFIS CATERING NO 8236, JLN BUNGA RAYA 2, -

Eleventh Malaysia Plan 2016-2020 Anchoring GRowth on People
ELEVENTH MALAYSIA PLAN 2016-2020 ANCHORING G ROWTH ON PEOPLE ISBN 978-9675842085 For further information refer to: Director General, Economic Planning Unit, Prime Minister’s Department, Block B5 & B6, Federal Government Administrative Centre, 62502 Putrajaya. MALAYSIA. http://www.epu.gov.my email: [email protected] Tel.: 603-8000 8000 Fax: 603-8888 3755 Released on 21st May 2015 Reprinted on 29th May 2015 Publisher’s Copyright© All rights reserved. No part of this publication may be reproduced, copied, stored in any retrieval system or transmitted in any form or by any means – electronic, mechanical, photocopying, recording or otherwise; without prior permission of Economic Planning Unit, Prime Minister’s Department, Malaysia. Printed by Percetakan Nasional Malaysia Berhad, Kuala Lumpur, 2015 www.printnasional.com.my Email: [email protected] Tel: 03-92366895 Fax: 03-92224773 ELEVENTH MALAYSIA PLAN 2016-2020 ANCHORING G ROWTH ON PEOPLE Foreword Dato’ Sri Mohd Najib bin Tun Haji Abdul Razak Prime Minister of Malaysia i The Eleventh Malaysia Plan, 2016-2020, marks a momentous milestone in our nation’s history. With 2020 now just five years away, the Eleventh Plan is the next critical step in our journey to become an advanced nation that is inclusive and sustainable. In the last five years, although Malaysia encountered headwinds from a global economic slowdown, our economy has done extremely well with GDP growth among the fastest in the region. The quality of life of the rakyat has also improved as reflected by the increase in both per capita income and the average household income. This was made possible by the numerous reforms that were put in place by the Government to improve the quality of life of the people. -
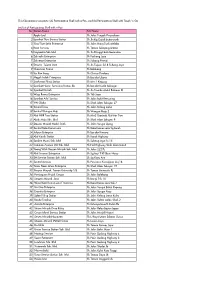
This File Contains Two Parts: (A) Participating Shell with E-Pay, and (B) Participating Shell with Touch 'N Go
This file contains two parts: (A) Participating Shell with e-Pay, and (B) Participating Shell with Touch 'n Go (A) List of Participating Shell with e-Pay No Station Name Site Name 1 Apple Leaf Sh Jalan Tengah Perusahaan 2 Syarikat Thye Service Station Sh Jln Kg Gajah Butterworth 3 Eng Thye Setia Enterprise Sh Jalan Hang Tuah Melaka 4 Reza Services Sh Taman Selayang Utama 5 Dayapetro Sdn Bhd Sh Jln Pringgit Batu Berendam 6 Zahiedin Enterprise Sh Puchong Jaya 7 Zahienor Enterprise Sh Subang Permai 8 Stesyen Tujuan Jaya Sh Jln Tujuan Ss18 Subang Jaya 9 Chop Lian Seong Sh Balakong 10 Sin Kee Sang Sh Cheras Perdana 11 Megah Indah Enterprise Sh Bandar Utama 12 Saaharaa Filing Station Sh Mrr 2 Kepong 13 Syarikat Henry Servicing Station SB Sh Bandar Kuala Selangor 14 Syarikat Durrah Sh Jln Tuanku Abdul Rahman Kl 15 Waja Reena Enterprise Sh Ttdi Jaya 16 Syarikat Arbi Service Sh Jalan Bukit Kemuning 17 YW Global Sh Shah Alam Seksyen 27 18 Sentral Tiraz Sh Jalan Kelang Lama 19 Sentral Wangsa Maju Sh Wangsa Maju 2 20 Alaf MRR Two Station Sh Mrr2 Gombak Alaf Mrr Two 21 Abah Maju Sdn. Bhd. Sh Shah Alam Seksyen 9 22 Stesyen Minyak Mohd. Diah Sh Jalan Sungai Ujong 23 Sentral Kota Damansara Sh Kota Damansara Sg Buloh 24 Jufiyun Enterprise Sh Bandar Kinrara 25 Alaf Karak Station Sh Karak Highway 26 Spektra Murni Sdn. Bhd. Sh Subang Jaya Ss 15 27 Common Avenue (M) Sdn. Bhd. Sh Fed Highway Shah Alam Batu3 28 Yeong Wah Stesyen Minyak Sdn. -

Vol. 38 (2) May 2015 I 1 21 235 259 219 175 187 197 161
Pertanika JTAS Pertanika Pertanika Journal of Tropical Agricultural Science Vol. 38 (2) May 2015 Contents Foreword Nayan Deep S. Kanwal i Review Article Application of PCR and MAS: Potential Use for Assessment of Genetic 161 Diversity of Rice Germplasm in Breeding Programmes in Developing Countries Wijerathna, Y. M. A. M., Perera, A. N. K., Hamama, I. B. and Hoang, L. Regular Articles Effects of Combination Ethephon and Urea on Productivity of 175 Jatropha curcas L. Thai Accession Shuib, N. H. and Sulaiman, Z. Effective Elements on Technical Knowledge of Agricultural Section for 187 Vol. 38 (2) May 2015 38 (2) May Vol. Sustainable Soil Management Mohammad Sadegh Sabouri, Meysam Solouki and Marzieh Bordbar Ethnomedicinal Study of Plants in Hathazari, Chittagong, Bangladesh 197 Sajib, N. H. and Uddin, S. B. Length-Weight Relationship and Relative Condition Factor of 211 Parapenaeopsis sculptilis (Heller, 1862) from the Coastal Waters of Perak, Peninsular Malaysia Amani A. A., Arshad, A., Yusoff, F. M. and Amin, S. M. N. Dissemination of Natural Resource Management Technology for Irrigated 219 Rice in the Philippines: On-Farm Validation to National Extension Corales, A. M., Sibayan, E. B. and Palis, F. G. Isolation and Characterization of EgGST, a Glutathione S-transferase 235 Protein Transcript in Oil Palm (Elaeis guineensis Jacq.) Conie Toh, Parameswari Namasivayam, Ho Chai Ling and Sharifah Shahrul Rabiah Syed Alwee VOL. 38 (2) MAY 2015 Optimisation of Solid Liquid Extraction of Orthosiphon stamineus 259 Leaves using Response Surface Methodology -

TROPICAL AGRICULTURAL SCIENCE Optimisation Of
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Universiti Putra Malaysia Institutional Repository Pertanika J. Trop. Agric. Sci. 38 (2): 259 - 270 (2015) TROPICAL AGRICULTURAL SCIENCE Journal homepage: http://www.pertanika.upm.edu.my/ Optimisation of Solid Liquid Extraction of Orthosiphon stamineus Leaves using Response Surface Methodology Technique Mohd Farhan, A. R.1*, Pin, K. Y.2, Zamree, M. S.2, Luqman Chuah, A.3 and Nazira, M.1 1Faculty of Industrial Science and Technology, Universiti Malaysia Pahang, Tun Razak Highway, 26300 Gambang, Kuantan, Pahang, Malaysia 2Natural Product Division, Forest Research Institute Malaysia, 52109 Kepong, Selangor, Malaysia 3Department of Chemical and Environmental, Faculty of Engineering, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia ABSTRACT Orthosiphon stamineus is one of the popular medicinal plants in Southeast Asia. O. stamineus leaves are used in numerous applications related to medicinal purposes and are believed to cure certain health conditions such as hypertension, gout and fever. The aim of this study was to investigate the effect of three parameters involved in extraction process including extraction temperature, extraction duration and solvent to solid ratio on extraction yield, antioxidant activity and referral markers of O. stamineus leaves. The optimisation of extraction processes was evaluated with the aid of Design-Expert software using response surface methodology (RSM). The optimum extraction parameter for O. stamineus leaves were recorded at the extraction temperature of 60°C, 30:1 (ml:g) solvent to solid ratio and 6 hours extraction duration with 30Wt% extract, 67 and 1 mg/L concentration of Rosmarinic acid and Sinensetin, respectively. -
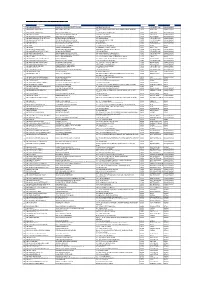
Shell Lebih Ekstra at Zalora Promotion Participating Stations List NO SITE
Shell Lebih Ekstra at Zalora Promotion Participating Stations List NO SITE NAME STATION NAME ADDRESS POSCODE CITY STATE 1 SH JALAN JELUTONG BAN LEONG SHELL PRODUCTS SDN BHD 347 JELUTONG ROAD 11600 GEORGETOWN PULAU PINANG 2 SH BANDAR AYER ITAM 2 BBAI SHELL SERVICES LOT 2499 JALAN THEAN TEIK, JALAN SHAIK MADAR BANDAR 11500 AYER ITAM PULAU PINANG BARU 3 SH BANDAR AYER ITAM 1 BBAI SALES & SERVICES 12 ANGSANA FARLIM ROAD 11500 AYER ITAM PULAU PINANG 4 SH BUKIT GELUGOR BUKIT GLUGOR SERVICE STATION 210 BUKIT GELUGOR 11700 GELUGOR PULAU PINANG 5 SH JLN MAYANG PASIR BAYAN BARU CERGAS SAUJANA SDN BHD JALAN MAYANG PASIR 11950 BAYAN BARU PULAU PINANG 6 SH JALAN BURMAH GEORGE TOWN ELITEBAY EXPRESS ENTERPRISE 378 JALAN BURMA 10350 GEORGETOWN PULAU PINANG 7 SH JALAN MESJID NEGERI GREEN ISLAND SERVICE STATION 4A JALAN MASJID NEGERI 11600 GEORGETOWN PULAU PINANG 8 SH BALIK PULAU KEAN YOON FATT FILLING STATION 315 GENTING 11000 BALIK PULAU PULAU PINANG 9 SH WELD QUAY LEAN HONG CO SDN BHD 30 WELD QUAY 10300 GEORGETOWN PULAU PINANG 10 SH GERIK MAESTRO ONE ENTERPRISE 122 JLN SULTAN ISKANDAR 33300 GERIK PERAK 11 SH LAWIN MEERA AAZ ENTERPRISE 2B KAMPUNG MALAU, LAWIN 33410 LENGGONG PERAK 12 SH JELUTONG EXPRESSWAY MILYAR MUTIARA ENTERPRISE LEBUHRAYA TUN DR LIM CHONG EU 11600 GEORGETOWN PULAU PINANG 13 SH JALAN PERAK GEORGE TOWN MS MASHA ENTERPRISE 190 JALAN PERAK 10150 GEORGETOWN PULAU PINANG 14 SH JALAN KELAWEI BIRCH MUKAH HEAD SERVICE STATION 2A JALAN KELEWAI / JALAN BIRCH 10250 GEORGETOWN PULAU PINANG 15 SH JALAN PAYA TERUBONG PAYA TERUBONG SERVICE STATION -

Food Quality Assurance of Crude Palm Oil: a Review on Toxic Ester Feedstock
OCL 2021, 28, 23 © A.F.A. Nizam and M.S. Mahmud, Hosted by EDP Sciences, 2021 https://doi.org/10.1051/ocl/2021011 OCL Oilseeds & fats Crops and Lipids Available online at: www.ocl-journal.org REVIEW Food quality assurance of crude palm oil: a review on toxic ester feedstock Ainul Farhani Ahmad Nizam and Mohd Sabri Mahmud* Department of Chemical Engineering, College of Engineering, Universiti Malaysia Pahang, Tun Razak Highway, Gambang, 26300 Kuantan, Pahang, Malaysia Received 16 April 2020 – Accepted 8 March 2021 Abstract – Palm oil, the commodity produced mainly in Indonesia and Malaysia, is widely used for deep- frying of fast food and food derivatives. European and American markets of palm oil are affected by the concern of the toxicity potential from monochloropropanediol esters (MCPDE) and glycidyl ester (GE) that are undesirably produced from monoacylglycerol (MAG), diacylglycerol (DAG) and chlorine in refineries. Improvement of oil palm plantation, fruit harvest and oil extraction process in palm oil mills is necessary before the refinery process so that hydrolysis reactions that produce MAG and DAG and chlorine contamination can be minimized in crude palm oil (CPO). This review focuses on the quality control currently employed in the mills especially in managing free fatty acid (FFA) formation as the indicator of the hydrolysis reactions along with other quality control parameters and the reduction of chlorine content. Keywords: monochloropropanediol / glycidyl esters / crude palm oil / food quality / food contaminant Résumé – Assurance qualité alimentaire de l’huile de palme brute : un examen des matières premières à base d’esters toxiques. L’huile de palme, produite principalement en Indonésie et en Malaisie, est largement utilisée pour la friture des aliments de restauration rapide et de leurs dérivés. -

COVER-Geo Capability Statement
GEOTECHNICAL CAPABILITY STATEMENT e u q G i e n n e U r > a QUALITY t e SYSTEM QUALITY i v v MANAGEMENT e t i SIRIM > C r e a MS ISO 9001 : 2000 REG. NO. AR 4335 GCU Capability Statement GCU KL Office (Main) GCU Consultants Sdn Bhd 3F-09, IOI Business Park, Bandar Puchong Jaya, 47170 Puchong, Selangor Darul Ehsan Tel : +603 – 8070 5501 Fax : +603 – 8070 5503 Contact Person : Ir. EG Balakrishnan / Ir. Mohd Redzuan H/P No : +6012 – 201 0434 / 019 – 281 3018 C&S Office GCU Consultants (C&S) Sdn Bhd 1F-28, IOI Business Park, Bandar Puchong Jaya, 47170 Puchong, Selangor Darul Ehsan Tel : +603 – 8070 5502 Fax : +603 – 8070 5503 Contact Person : Ir. S Vijayakumar / Ir. R Thinagaran H/P No : +6012 – 390 0966 / 012 – 385 6706 M&E Office GCU Consultants (M&E) Sdn Bhd E3A-03A, Neo Damansara, Jalan PJU 8/11, Bandar Damansara Perdana, 47820 Petaling Jaya, Selangor Darul Ehsan Contact Person : Ir. Narendran Naidu H/P No : +6012 – 278 7974 Fax : +603 – 7729 0654 Johor Office GCU Consultants (Johor) Sdn Bhd No.12-02, Jalan Molek 1/5C, Taman Molek, 81100 Johor Bahru, Johor Darul Takzim Tel : +607 – 350 7391 Fax : +607 – 350 7392 Contact Person : Ir. Lee Kai Ming H/P No : +6012 – 232 3806 Sabah Office GCU Consultants (Sabah) Sdn Bhd Lot B-11A-2, Block B, Pavilion Bundusan, Lorong Pavilion Bundusan 3, Jalan Bundusan, 88300 Penampang, Sabah Tel / Fax : +6088 – 739 755 Contact Person : Ir. Amarjit Singh H/P No : +6017 – 339 8479 Y:\1.0 CAPABILITY STATEMENT\1. -

Business Name Business Category Outlet Address State 2M Automotive
Business Name Business Category Outlet Address State 2M Automotive Automotive 22 GROUND FLOOR JALAN DATO SHEIKH AHMAD SEREMBAN NEGERI SEMBILAN70000 Negeri Sembilan Abul Kalam enterprise Automotive Abul Kalam enterprise no 25 Jalan 3/1 Nilai3 Negeri Sembilan Aby Tyre Centre Automotive LOT 687 KG SASAPAN BATU REMBAU BERANANG Negeri Sembilan ADIB SUCCESS GARAGE Automotive TANJONG IPOH NO 32 Tanjung Ipoh Negeri Sembilan Malaysia Negeri Sembilan Ah Keong Motor Service Automotive 138139 jalan tampin 4 1/2 miles senawang light industrial area 70450 seremban Negeri sembilan Jalan Tampin Seremban Negeri Sembilan Malaysia Negeri Sembilan Ah Keong Motor Svc Automotive LOT 138 & 139, SENAWANG LIGHT IND AREA, BT 4 1/2, JALAN TAMPIN 70450, SEREMBAN NEGERI SEMBILAN70450 Negeri Sembilan AJ AVENUE CAR WASH Automotive NO 21 JALAN HARTAMAS 2 Jalan Seremban Taman Port Dickson Port Dickson Negeri Sembilan Malaysia Negeri Sembilan alat alat ganti kenderaan Automotive Jalan Selandar Gemencheh Gemencheh Negeri Sembilan Malaysia Negeri Sembilan AMIR IMRAN ENTERPRISE Automotive Imran Carpets205 JALAN 3/6 NILAI 3 Lot 205 Jalan Nilai 3/6 Kawasan Perindustrian Nilai 3 Nilai Negeri Sembilan Malaysia Negeri Sembilan ARH Auto Care Seremban Automotive No.93 Jalan MSJ 4,Medan Perniagaan SenawangNegeri Sembilan Negeri Sembilan Arh Auto Care Sg Gadut Automotive No 41G, Jalan TJ 1/4A,Kawasan Perindustrian Tuanku Jaafar, Sungai Gadut,Negeri Sembilan Negeri Sembilan Asia Access Parking Automotive C/O Asia Access Parkig No10 Jalan Wolff Seremban Negeri Sembilan Malaysia Negeri -

Asian Toll Road Development Program (1999)
The World Bank Ministry of Construction, Japan Asian Toll Road Development Program Review of Recent Toll Road Experience in Selected Countries and Preliminary Tool Kit for Toll Road Development Draft Final Report May 1999 in association with Highway Planning Inc., and Value Management Institute, Inc. ACKNOWLEDGMENTS This report was prepared based on a study financed by the World Bank for the Asian Toll Road Development Program. It was prepared under the direction of the World Bank Steering Committee chaired by Alfred Nickesen; the Steering Committee of the Ministry of Construction, Japan, headed by Junichi Matoba; and the Japanese Advisory Committee headed by Prof. Yataro Fujii. They have all provided valuable assistance and comments to the authors during the study period. The authors also would like to acknowledge various others including Setsuo Hirai of the World Bank, the Task Leader of this project; the advisory team of the Expressway Technology Center and the Express Highway Research Foundation headed by Yoshimichi Kawasumi; and Mitchel Stanfield of MSA. Their assistance in information collection and comments on the earlier drafts has been helpful. Finally, the authors wish to extend their gratitude to a number of individuals who kindly shared their valuable experience and insights with us during the country visits to the Hong Kong Special Administrative Region, Singapore, Malaysia, Indonesia, Thailand, and the Philippines. A complete list of the members of the Steering and Advisory Committees mentioned above, and a list of individuals who were interviewed by the consultants are included in the Appendicies of this report. The primary authors of this report were Chiaki Kuranami, Bruce P. -

SGS RSPO Doc
SGS RSPO Doc. Number: GP 7003A (Associated Document) Doc. Version date: 01 January 2013 Page: 1 of 61 OIL PALM PLANTATION MANAGEMENT VERIFICATION REPORT Report Nr: MY02410-Boustead Plantations Client: Boustead Rimba Nilai Sdn Bhd (346457-W) RSPO 1-0012-04-000-00 membership #: www.boustead.com.my Web Page: Address: 28th Floor, Menara Boustead 69, Jalan Raja Chulan, 50200 Kuala Lumpur Malaysia Telephone: 603-2145-2121 Country: Malaysia Certification Sg Jernih Business Unit Unit Evaluated: Total Plantation 7,029 hectares Area Company En Mohammad Tarmizi Taufek Contact Person: Head office: Mill: 11h Floor, Menara Boustead, Kilang Kelapa Sawit Sungai Jernih Address: 69 Jalan Raja Chulan, Wakil Pos Paloh Inai 50200 Kuala Lumpur, 26650 Pekan, Pahang Malaysia Malaysia Tel: 03-2145-2121 Fax 03-2141-1690 Email: [email protected] Evaluation dates: Pre-assessment 26 –28 April 2010 Main 21 – 25 March 2011 Assessment 1st Surveillance 07-10 August 2012 GP 7003A Page 2 of 61 TABLE OF CONTENTS LIST OF TABLES .................................................................................................................................................... 3 LIST OF MAPS ........................................................................................................................................................ 3 LIST OF FIGURES ..................................................................................... ERROR! BOOKMARK NOT DEFINED. LIST OF PHOTOS ..................................................................................... -

Thermodynamic Performance Analysis of Gas-Turbine Power-Plant
International Journal of the Physical Sciences Vol. 6(14), pp. 3539-3550, 18 July, 2011 Available online at http://www.academicjournals.org/IJPS DOI: 10.5897/IJPS11.272 ISSN 1992 - 1950 ©2011 Academic Journals Full Length Research Paper Thermodynamic performance analysis of gas-turbine power-plant M. M. Rahman 1,2 , Thamir K. Ibrahim 1* and Ahmed N. Abdalla 3 1Faculty of Mechanical Engineering, Universiti Malaysia Pahang, Tun Razak Highway, 26300 Gambang, Kuantan, Pahang, Malaysia 2Automotive Engineering Centre, Universiti Malaysia Pahang, Tun Razak Highway, 26300 Gambang, Kuantan, Pahang, Malaysia 3Faculty of Electrical and Electronic Engineering, Universiti Malaysia Pahang, Tun Razak Highway, 26300 Gambang, Kuantan, Pahang, Malaysia. Accepted 04 April, 2011 This paper was presented the parametric study of thermodynamic performance on gas turbine power plant. The variation of operating conditions (compression ratio, turbine inlet and exhaust temperature, air to fuel ratio, isentropic compressor and turbine efficiency, and ambient temperature) on the performance of gas turbine (thermal efficiency, compressor work, power, specific fuel consumption, heat rate) were investigated. The analytical formula for the specific work and efficiency were derived and analyzed. The programming of performance model for gas turbine was developed utilizing the MATLAB software. The results show that the compression ratio, ambient temperature, air to fuel ratio as well as the isentropic efficiencies are strongly influence on the thermal efficiency. In addition, the thermal efficiency and power output decreases linearly with increase of the ambient temperature and air to fuel ratio. However, the specific fuel consumption and heat rate increases linearly with increase of both ambient temperature and air to fuel ratio.