Cell Structure and Protein Secretion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cytoplasmic Organelles
CYTOPLASMIC ORGANELLES OBJECTIVES: After completing this exercise, you should be able to: 1. Recognize features of the cytoplasm in the light microscope. 2. Identify major cellular organelles in electron micrographs. 3. Provide general functions of cellular organelles. ASSIGNMENT FOR TODAY'S LABORATORY GLASS SLIDES - https://medmicroscope.uc.edu/ SL 181 (spinal cord) rough endoplasmic reticulum SL 108 (pancreas) rough endoplasmic reticulum SL 125 (inflammation) rough endoplasmic reticulum and Golgi apparatus ELECTRON MICROGRAPHS (Gray envelope) EM 3-6, 4-5, 12-1, 16 plasma membrane EM 1-3, 2-1, 2-2, 3-5, 4-1, 10-5, 14-3 rough endoplasmic reticulum EM 4-2, 10-6, 12-3, 13-7, 14-5 smooth endoplasmic reticulum EM 5-2 and 5-inset ribosomes EM 11-1 to 11-4 Golgi apparatus EM 6-10 to 6-13, 2-3 to 2-5 also 3-4, 1-4, 12-2 and 13-6 mitochondria EM 3, 4, 16 and 17 Magnification and Resolution POSTED ELECTRON MICROGRAPHS # 1 Organelles # 6 Organelles Lab 2 Posted EMs SUPPLEMENTAL MATERIAL: SUPPLEMENTARY ELECTRON MICROGRAPHS Rhodin, J. A.G., An Atlas of Histology Plasma membrane Fig. 2-2; 2-3 Rough ER Fig 2-26; 2-29; 2-30; 2-31; 2-32 Smooth ER Fig 2-33; 2-34; 2-35 Ribosomes Fig 2-27; 2-28; 2-30; 2-31; 2-32 Golgi apparatus Fig 2-36; 2-37; 2-38 Mitochondria Fig 2-39; 2-40; 2-41 In the last lab, you were introduced to cells and extracellular matrix, followed by a focus on the nucleus. -

A Tour of the Cell Overview
Unit 3: The Cell Name______________________ Chapter 6: A Tour of the Cell Overview 6.1 Biologists use microscopes and the tools of biochemistry to study cells The discovery and early study of cells progressed with the invention of microscopes in 1590 and their improvement in the 17th century. In a light microscope (LM), visible light passes through the specimen and then through glass lenses. ○ The lenses refract light so that the image is magnified into the eye or a camera. Microscopes vary in magnification, resolution, and contrast. ○ Magnification is the ratio of an object’s image to its real size. A light microscope can magnify effectively to about 1,000 times the real size of a specimen. ○ Resolution is a measure of image clarity. It is the minimum distance two points can be separated and still be distinguished as two separate points. The minimum resolution of an LM is about 200 nanometers (nm), the size of a small bacterium. ○ Contrast accentuates differences in parts of the sample. It can be improved by staining or labeling of cell components so they stand out. Although an LM can resolve individual cells, it cannot resolve much of the internal anatomy, especially the organelles, membrane-enclosed structures within eukaryotic cells. The size range of cells 1 To resolve smaller structures, scientists use an electron microscope (EM), which focuses a beam of electrons through the specimen or onto its surface. ○ Theoretically, the resolution of a modern EM could reach 0.002 nm, but the practical limit is closer to about 2 nm. Scanning electron microscopes (SEMs) are useful for studying the surface structure or topography of a specimen. -

Protein Export Via the Type III Secretion System of the Bacterial Flagellum
biomolecules Review Protein Export via the Type III Secretion System of the Bacterial Flagellum Manuel Halte and Marc Erhardt * Institute for Biology–Bacterial Physiology, Humboldt-Universität zu Berlin, Philippstr. 13, 10115 Berlin, Germany; [email protected] * Correspondence: [email protected] Abstract: The bacterial flagellum and the related virulence-associated injectisome system of pathogenic bacteria utilize a type III secretion system (T3SS) to export substrate proteins across the inner membrane in a proton motive force-dependent manner. The T3SS is composed of an export gate (FliPQR/FlhA/FlhB) located in the flagellar basal body and an associated soluble ATPase complex in the cytoplasm (FliHIJ). Here, we summarise recent insights into the structure, assembly and protein secretion mechanisms of the T3SS with a focus on energy transduction and protein transport across the cytoplasmic membrane. Keywords: bacterial flagellum; flagellar assembly; type III protein export; ATPase; proton motive force; secretion model 1. Introduction Flagella are complex rotary nanomachines embedded in the cell envelope of many Citation: Halte, M.; Erhardt, M. bacteria. In addition to functions in adhering to surfaces, flagella allow bacteria to move Protein Export via the Type III in their environment towards nutrients or to escape harmful molecules. They are present Secretion System of the Bacterial in both Gram-negative and Gram-positive bacteria, and are evolutionary related to the Flagellum. Biomolecules 2021, 11, 186. injectisome device, which various Gram-negative bacterial species use to inject effectors into https://doi.org/10.3390/ eukaryotic target cells [1]. Both the flagellum and injectisome are complex nanomachines biom11020186 and made of around 20 different proteins, ranging from a copy number of very few to several thousand [2]. -

A Study of Extracellular Space in Central Nervous Tissue by Freeze-Substitution
A STUDY OF EXTRACELLULAR SPACE IN CENTRAL NERVOUS TISSUE BY FREEZE-SUBSTITUTION A. VAN HARREVELD, M.D., JANE CROWELL, Ph.D., and S. K. MALHOTRA, D.Phil. From the Kerckhoff Laboratories of the Biological Sciences, California Institute of Technology, Pasadena, California ABSTRACT Downloaded from It was attempted to preserve the water distribution in central nervous tissue by rapid freezing followed by substitution fixation at low temperature. The vermis of the cerebellum of white mice was frozen by bringing it into contact with a polished silver mirror maintained at a temperature of about -207C. The tissue was subjected to substitution fixation in acetone containing 2 per cent Os0 4 at -85°C for 2 days, and then prepared for electron micros- copy by embedding in Maraglas, sectioning, and staining with lead citrate or uranyl www.jcb.org acetate and lead. Cerebellum frozen within 30 seconds of circulatory arrest was compared with cerebellum frozen after 8 minutes' asphyxiation. From impedance measurements under these conditions, it could be expected that in the former tissue the electrolyte and water distribution is similar to that in the normal, oxygenated cerebellum, whereas in the on August 22, 2006 asphyxiated tissue a transport of water and electrolytes into the intracellular compartment has taken place. Electron micrographs of tissue frozen shortly after circulatory arrest re- vealed the presence of an appreciable extracellular space between the axons of granular layer cells. Between glia, dendrites, and presynaptic endings the usual narrow clefts and even tight junctions were found. Also the synaptic cleft was of the usual width (250 to 300 A). -
Lysosome Trafficking Is Necessary for EGF-Driven Invasion and Is
Dykes et al. BMC Cancer (2017) 17:672 DOI 10.1186/s12885-017-3660-3 RESEARCH ARTICLE Open Access Lysosome trafficking is necessary for EGF- driven invasion and is regulated by p38 MAPK and Na+/H+ exchangers Samantha S. Dykes1,2,4, Joshua J. Steffan3* and James A. Cardelli1,2 Abstract Background: Tumor invasion through a basement membrane is one of the earliest steps in metastasis, and growth factors, such as Epidermal Growth Factor (EGF) and Hepatocyte Growth Factor (HGF), stimulate this process in a majority of solid tumors. Basement membrane breakdown is one of the hallmarks of invasion; therefore, tumor cells secrete a variety of proteases to aid in this process, including lysosomal proteases. Previous studies demonstrated that peripheral lysosome distribution coincides with the release of lysosomal cathepsins. Methods: Immunofluorescence microscopy, western blot, and 2D and 3D cell culture techniques were performed to evaluate the effects of EGF on lysosome trafficking and cell motility and invasion. Results: EGF-mediated lysosome trafficking, protease secretion, and invasion is regulated by the activity of p38 mitogen activated protein kinase (MAPK) and sodium hydrogen exchangers (NHEs). Interestingly, EGF stimulates anterograde lysosome trafficking through a different mechanism than previously reported for HGF, suggesting that there are redundant signaling pathways that control lysosome positioning and trafficking in tumor cells. Conclusions: These data suggest that EGF stimulation induces peripheral (anterograde) lysosome trafficking, which is critical for EGF-mediated invasion and protease release, through the activation of p38 MAPK and NHEs. Taken together, this report demonstrates that anterograde lysosome trafficking is necessary for EGF-mediated tumor invasion and begins to characterize the molecular mechanisms required for EGF-stimulated lysosome trafficking. -

Reconstructions of Centriole Formation and Ciliogenesis in Mammalian Lungs
J. Cell Sci. 3, 207-230 (1968) 207 Printed in Great Britain RECONSTRUCTIONS OF CENTRIOLE FORMATION AND CILIOGENESIS IN MAMMALIAN LUNGS S. P. SOROKIN Department of Anatomy, Harvard Medical School, Boston, Massachusetts 02115, U.S.A. SUMMARY This study presents reconstructions of the processes of centriolar formation and ciliogenesis based on evidence found in electron micrographs of tissues and organ cultures obtained chiefly from the lungs of foetal rats. A few observations on living cultures supplement the major findings. In this material, centrioles are generated by two pathways. Those centrioles that are destined to participate in forming the achromatic figure, or to sprout transitory, rudimentary (primary) cilia, arise directly off the walls of pre-existing centrioles. In pulmonary cells of all types this direct pathway operates during interphase. The daughter centrioles are first recognizable as annular structures (procentrioles) which lengthen into cylinders through acropetal deposition of osmiophilic material in the procentriolar walls. Triplet fibres develop in these walls from singlet and doublet fibres that first appear near the procentriolar bases and thereafter extend apically. When little more than half grown, the daughter centrioles are released into the cyto- plasm, where they complete their maturation. A parent centriole usually produces one daughter at a time. Exceptionally, up to 8 have been observed to develop simultaneously about 1 parent centriole. Primary cilia arise from directly produced centrioles in differentiating pulmonary cells of all types throughout the foetal period. In the bronchial epithelium they appear before the time when the ciliated border is generated. Fairly late in foetal life, centrioles destined to become kinetosomes in ciliated cells of the epithelium become assembled from masses of fibrogranular material located in the apical cytoplasm. -

Intracellular Transport in Eukaryotes
Intracellular transport in eukaryotes Overview Compartmentalization and inner membranes enables eukaryotic cells • to be 1000-10000 times larger than prokaryotes • to isolate specialized chemical processes in specific parts of the cell • to produce “packages” (vesicles) of chemical components that can be shuttled around the cell actively Membrane-enclosed organelles take up ~50% of the volume of eukaryotic cells: • nucleus – genomic function • endoplasmic reticulum – synthesis of lipids; on the border with the cytosol, synthesis of proteins destined for many organelles and the plasma membrane • Golgi apparatus – modification, sorting, and packaging of proteins and lipids for specific intracellular destination (akin to a mail sort facility) • lysosomes – degradation • endosomes – sorting of endocytosed (engulfed) material by the cell • peroxisomes – oxidation of toxic species • mitochondria , chloroplasts – energy conversion Cells contain ͥͤ ͥͦ protein molecules that are constantly being synthesized and 10 Ǝ 10 degraded Proteins are synthesized in the cytosol , but not all proteins remain there and many must be transported to the appropriate compartment For comparison: transport by diffusion Even without active transport requiring free energy transduction, movement of molecules in the cell is rapid by diffusive motion © M. S. Shell 2009 1/11 last modified 10/27/2010 Consider a sea of molecules. Pinpoint one molecule and note its starting position at time 0. Due to thermal motion, the particle on average makes a random jump of length every units ͠ of time. The jump is random in the radial direction. This is called a random walk . Repeat this process for many jumps n and interrogate the final distance of the particle from its starting point ͦ ͠ We could imagine doing many such experiments. -

Membrane Structure in Mammalian Astrocytes: a Review of Freeze-Fracture Studies on Adult, Developing, Reactive and Cultured Astrocytes
y. exp. Bid. (1981), 95. 35~48 35 JVith 6 figures 'Printed in Great Britain MEMBRANE STRUCTURE IN MAMMALIAN ASTROCYTES: A REVIEW OF FREEZE-FRACTURE STUDIES ON ADULT, DEVELOPING, REACTIVE AND CULTURED ASTROCYTES BY DENNIS M. D. LANDIS Department of Neurology, Massachusetts General Hospital, Boston, MA. 02114 AND THOMAS S. REESE Section on Functional Neuroanatomy, National Institute of Neurological and Communicative Diseases and Stroke, National Institutes of Health, Bethesda, MD. 20014 SUMMARY The application of freeze-fracture techniques to studies of brain structure has led to the recognition of two unsuspected specializations of membrane structure, each distributed in a specific pattern across the surface of astro- cytes. 'Assemblies' (aggregates of uniform, small particles packed in orthogonal array into rectangular or square aggregates) are found to characterize astrocytic plasma membranes apposed to blood vessels or to the cerebrospinal fluid at the surface of the brain. These particle aggregates are much less densely packed in astrocytic processes in brain parenchyma. Assemblies are not fixation artifacts, have been shown to extend to the true outer surface of the membrane, are remarkably labile in the setting of anoxia, and are at least in part protein. The function of assemblies is unknown, but their positioning suggests that they may have a role in the transport of some material into or out of the blood and cerebrospinal fluid compartments. A second specialization of intramembrane particle distri- bution, the polygonal particle junction, links astrocytic processes at the surface of the brain, and also links proximal, large caliber astrocytic processes in brain parenchyma. The function of this membrane specialization also is unknown, but it may subserve a mechanical role. -

The Flagellum and Flagellar Pocket of Trypanosomatids
Molecular & Biochemical Parasitology 115 (2001) 1–17 www.parasitology-online.com. Reviews: Parasite cell Biology: 1 The flagellum and flagellar pocket of trypanosomatids Scott M. Landfear *, Marina Ignatushchenko Department of Molecular Microbiology and Immunology, Oregon Health Sciences Uni6ersity, Portland, OR 97201, USA Received 9 November 2000; received in revised form 26 January 2001; accepted 5 March 2001 Abstract The flagellum and flagellar pocket are distinctive organelles present among all of the trypanosomatid protozoa. Currently, recognized functions for these organelles include generation of motility for the flagellum and dedicated secretory and endocytic activities for the flagellar pocket. The flagellar and flagellar pocket membranes have long been recognized as morphologically separate domains that are component parts of the plasma membrane that surrounds the entire cell. The structural and functional specialization of these two membranes has now been underscored by the identification of multiple proteins that are targeted selectively to each of these domains, and non-membrane proteins have also been identified that are targeted to the internal lumina of these organelles. Investigations on the functions of these organelle-specific proteins should continue to shed light on the unique biological activities of the flagellum and flagellar pocket. In addition, work has begun on identifying signals or modifications of these proteins that direct their targeting to the correct subcellular location. Future endeavors should further refine our knowledge of targeting signals and begin to dissect the molecular machinery involved in transporting and retaining each polypeptide at its designated cellular address. © 2001 Elsevier Science B.V. All rights reserved. Keywords: Trypanosomatid protozoa; Flagellum; Flagellar Pocket; Organelle-specific proteins; Review 1. -
4.5 the Cytoskeleton
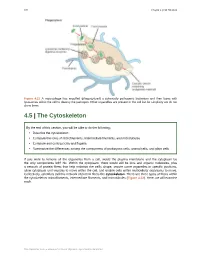
126 Chapter 4 | Cell Structure Figure 4.21 A macrophage has engulfed (phagocytized) a potentially pathogenic bacterium and then fuses with lysosomes within the cell to destroy the pathogen. Other organelles are present in the cell but for simplicity we do not show them. 4.5 | The Cytoskeleton By the end of this section, you will be able to do the following: • Describe the cytoskeleton • Compare the roles of microfilaments, intermediate filaments, and microtubules • Compare and contrast cilia and flagella • Summarize the differences among the components of prokaryotic cells, animal cells, and plant cells If you were to remove all the organelles from a cell, would the plasma membrane and the cytoplasm be the only components left? No. Within the cytoplasm, there would still be ions and organic molecules, plus a network of protein fibers that help maintain the cell's shape, secure some organelles in specific positions, allow cytoplasm and vesicles to move within the cell, and enable cells within multicellular organisms to move. Collectively, scientists call this network of protein fibers the cytoskeleton. There are three types of fibers within the cytoskeleton: microfilaments, intermediate filaments, and microtubules (Figure 4.22). Here, we will examine each. This OpenStax book is available for free at http://cnx.org/content/col24361/1.8 Chapter 4 | Cell Structure 127 Figure 4.22 Microfilaments thicken the cortex around the cell's inner edge. Like rubber bands, they resist tension. There are microtubules in the cell's interior where they maintain their shape by resisting compressive forces. There are intermediate filaments throughout the cell that hold organelles in place. -

Role of Microtubules in the Distribution of the Golgi Apparatus
Proc. Nati Acad. Sci. USA Vol. 80, pp. 4286-4290, July 1983 Biochemistry Role of microtubules in the distribution of the Golgi apparatus: Effect of taxol and microinjected anti-a-tubulin antibodies (organelle organization/cytoskeleton) JURGEN WEHLAND*, MARYANNA HENKARTt, RICHARD KLAUSNERt, AND IGNACIO V. SANDOVALt§ *Laboratory of Molecular Biology and tImmunology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20205; and *Laboratory of Biochemistry and Metabolism, National Institute of Arthritis, Diabetes, and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland 20205 Communicated by Gilbert Ashwell, April 21, 1983 ABSTRACT Immunofluorescence microscopy reveals that both organization and distribution of both microtubules and the Gol- microtubule organizing center (MTOC) and Golgi apparatus are gi apparatus were studied by dual indirect immunofluores- contained in the same perinuclear area of A549 cells in inter- cence microscopy as described (6). Cells were permeabilized by phase. The cells display long microtubules stretching radially from immersion in cold methanol (-20°C) for 2 min. The Golgi ap- the MTOC to the plasma membrane. Treatment of cells with taxol paratus was studied by using a rabbit monospecific antibody results in polymerization of microtubules without relation to the was MTOC and formation of microtubule bundles predominantly lo- raised against the Golgi enzyme ,B-galactosyltransferase and calized in the cell periphery. After incubation with taxol, the -

Immunocytochemical Localization of a Chondroitin Sulfate Proteoglycan in Nervous Tissue
Immunocytochemical Localization of a Chondroitin Sulfate Proteoglycan in Nervous Tissue. II. Studies in Developing Brain D. A. AQUINO, R. U. MARGOLIS, and R. K. MARGOLIS Downloaded from http://rupress.org/jcb/article-pdf/99/3/1130/1390417/1130.pdf by guest on 26 September 2021 Department of Pharmacology, New York University Medical Center, New York 10016; and Department of Pharmacology, State University of New York, Downstate Medical Center, Brooklyn, New York 11203 ABSTRACT In contrast to the intracellular (cytoplasmic) localization of chondroitin sulfate proteoglycans in adult brain (Aquino, D. A., R. U. Margolis, and R. K. Margolis, 1984, J. Cell Biol. 99:940-952), immunoelectron microscopic studies in immature (7 d postnatal) rat cerebellum demonstrated almost exclusively extracellular staining in the granule cell and molecular layers. Staining was also extracellular and/or associated with plasma membranes in the region of the presumptive white matter. Axons, which are unmyelinated at this age, generally did not stain, although faint intracellular staining was present in some astrocytes. At 10 and 14 d postnatal there was a significant decrease in extracellular space and staining, and by 21 d distinct cytoplasmic staining of neurons and astrocytes appeared. This intracellular staining further increased by 33 d so as to closely resemble the pattern seen in adult brain. Analyses of the proteoglycans isolated from 7-d-old and adult brain demonstrated that they have essentially identical biochemical compositions, immunochemical reactivity, size, charge, and density. These findings indicate that the antibodies used in this study recognize the same macromolecule in both early postnatal and adult brain, and that the localization of this proteoglycan changes progressively from an extracellular to an intracellular location during brain development.