Intralobar Fibres of the Occipital Lobe
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D
Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D. March 15, 2011 Anatomy Review • Lobes and layers • Brodmann’s areas • Vascular Supply • Major Neurological Findings – Frontal, Parietal, Temporal, Occipital, Limbic • Quiz Questions? Types of Cortex • Sensory • Motor • Unimodal association • Multimodal association necessary for language, reason, plan, imagine, create Structure of neocortex (6 layers) The general pattern of primary, association and mulimodal association cortex (Mesulam) Brodmann, Lateral Left Hemisphere MCA left hemisphere from D.Haines ACA and PCA -Haines Issues of Functional Localization • Earliest studies -Signs, symptoms and note location • Electrical discharge (epilepsy) suggested function • Ablation - deficit suggest function • Reappearance of infant functions suggest loss of inhibition (disinhibition), i.e. grasp, suck, Babinski • Variabilities in case reports • Linked networks of afferent and efferent neurons in several regions working to accomplish a task • Functional imaging does not always equate with abnormal function associated with location of lesion • fMRI activation of several cortical regions • Same sign from lesions in different areas – i.e.paraphasias • Notion of the right hemisphere as "emotional" in contrast to the left one as "logical" has no basis in fact. Limbic System (not a true lobe) involves with cingulate gyrus and the • Hippocampus- short term memory • Amygdala- fear, agression, mating • Fornix pathway to hypothalamus • -
Lateralization of Function Human Brain
Lateralization of Function Dr. Coulson Cognitive Science Department UCSD Human Brain • An extension of the spinal cord 1 Cerebral Hemispheres Corpus Callosum 2 Cartoon View of Brain Cerebral Lobes 3 Neurons • Brain composed of neurons – 100 billion • Neurons both send and receive signals to other cells in form of pulses • Important parts – Cell body –Axon – Synapse Connectivity • Each neuron connected to 10,000 other neurons • Point of contact is the synapse • Computing power of brain comes from connections 4 Cortex • Two millimeters thick and has area of 1.5 square meters Cartoon View: Frontal Lobe • In front of central sulcus • Decisions, judgments, emotions 5 Cartoon View: Parietal Lobe • Behind central sulcus • Perception of stimuli related to touch, pressure, temperature, pain Cartoon View: Temporal Lobe • Below lateral fissure • Perception, recognition, auditory processing 6 Cartoon View: Occipital Lobe • Located at back of brain, behind the parietal lobe and temporal lobe • Vision Lateralization of Function • One side of the brain is more crucial for a given function and/or more efficient at the underlying computational tasks • Typically a matter of degree – Strongly vs. Weakly Lateralized • Motor control a good example of a lateralized function 7 Sensorimotor Cortex 8 Motor Control What about language? • Language is a paradigmatic example of a lateralized cognitive phenomenon 9 Wada Test Lateralization of Function • Most evidence of lateralized brain function comes from observing how brain damage affects behavior on various sorts -

Cuneus and Fusiform Cortices Thickness Is Reduced in Trigeminal
Parise et al. The Journal of Headache and Pain 2014, 15:17 http://www.thejournalofheadacheandpain.com/content/15/1/17 RESEARCH ARTICLE Open Access Cuneus and fusiform cortices thickness is reduced in trigeminal neuralgia Maud Parise1,2*, Tadeu Takao Almodovar Kubo1, Thomas Martin Doring1, Gustavo Tukamoto1, Maurice Vincent1,3 and Emerson Leandro Gasparetto1 Abstract Background: Chronic pain disorders are presumed to induce changes in brain grey and white matters. Few studies have focused CNS alterations in trigeminal neuralgia (TN). Methods: The aim of this study was to explore changes in white matter microstructure in TN subjects using diffusion tensor images (DTI) with tract-based spatial statistics (TBSS); and cortical thickness changes with surface based morphometry. Twenty-four patients with classical TN (37-67 y-o) and 24 healthy controls, matched for age and sex, were included in the study. Results: Comparing patients with controls, no diffusivity abnormalities of brain white matter were detected. However, a significant reduction in cortical thickness was observed at the left cuneus and left fusiform cortex in the patients group. The thickness of the fusiform cortex correlated negatively with the carbamazepine dose (p = 0.023). Conclusions: Since the cuneus and the fusiform gyrus have been related to the multisensory integration area and cognitive processing, as well as the retrieval of shock perception conveyed by Aδ fibers, our results support the role of these areas in TN pathogenesis. Whether such changes occurs as an epiphenomenon secondary to daily stimulation or represent a structural predisposition to TN in the light of peripheral vascular compression is a matter of future studies. -

Structure and Function of Visual Area MT
AR245-NE28-07 ARI 16 March 2005 1:3 V I E E W R S First published online as a Review in Advance on March 17, 2005 I E N C N A D V A Structure and Function of Visual Area MT Richard T. Born1 and David C. Bradley2 1Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115-5701; email: [email protected] 2Department of Psychology, University of Chicago, Chicago, Illinois 60637; email: [email protected] Annu. Rev. Neurosci. Key Words 2005. 28:157–89 extrastriate, motion perception, center-surround antagonism, doi: 10.1146/ magnocellular, structure-from-motion, aperture problem by HARVARD COLLEGE on 04/14/05. For personal use only. annurev.neuro.26.041002.131052 Copyright c 2005 by Abstract Annual Reviews. All rights reserved The small visual area known as MT or V5 has played a major role in 0147-006X/05/0721- our understanding of the primate cerebral cortex. This area has been 0157$20.00 historically important in the concept of cortical processing streams and the idea that different visual areas constitute highly specialized Annu. Rev. Neurosci. 0.0:${article.fPage}-${article.lPage}. Downloaded from arjournals.annualreviews.org representations of visual information. MT has also proven to be a fer- tile culture dish—full of direction- and disparity-selective neurons— exploited by many labs to study the neural circuits underlying com- putations of motion and depth and to examine the relationship be- tween neural activity and perception. Here we attempt a synthetic overview of the rich literature on MT with the goal of answering the question, What does MT do? www.annualreviews.org · Structure and Function of Area MT 157 AR245-NE28-07 ARI 16 March 2005 1:3 pathway. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

The Neurobiology of Agrammatic Sentence Comprehension: a Lesion Study
The Neurobiology of Agrammatic Sentence Comprehension: A Lesion Study Corianne Rogalsky1, Arianna N. LaCroix1, Kuan-Hua Chen2,3, Steven W. Anderson2, Hanna Damasio4, Tracy Love5, and Gregory Hickok6 Abstract ■ Broca’s area has long been implicated in sentence compre- average did not exhibit the expected agrammatic compre- hension. Damage to this region is thought to be the central hension pattern—for example, their performance was >80% source of “agrammatic comprehension” in which performance on noncanonical sentences in the sentence–picture matching is substantially worse (and near chance) on sentences with non- task. Patients with ATL damage (n = 18) also did not exhibit canonical word orders compared with canonical word order an agrammatic comprehension pattern. Across our entire sentences (in English). This claim is supported by functional patient sample, the lesions of patients with agrammatic com- neuroimaging studies demonstrating greater activation in prehension patterns in either task had maximal overlap in pos- Broca’s area for noncanonical versus canonical sentences. terior superior temporal and inferior parietal regions. Using However, functional neuroimaging studies also have frequently voxel-based lesion–symptom mapping, we find that lower per- implicated the anterior temporal lobe (ATL) in sentence pro- formances on canonical and noncanonical sentences in each cessing more broadly, and recent lesion–symptom mapping task are both associated with damage to a large left superior studies have implicated the ATL and mid temporal regions in temporal–inferior parietal network including portions of the agrammatic comprehension. This study investigates these ATL, but not Broca’s area. Notably, however, response bias in seemingly conflicting findings in 66 left-hemisphere patients plausibility judgments was significantly associated with damage with chronic focal cerebral damage. -

Visual Cortex in Humans 251
Author's personal copy Visual Cortex in Humans 251 Visual Cortex in Humans B A Wandell, S O Dumoulin, and A A Brewer, using fMRI, and we discuss the main features of the Stanford University, Stanford, CA, USA V1 map. We then summarize the positions and proper- ties of ten additional visual field maps. This represents ã 2009 Elsevier Ltd. All rights reserved. our current understanding of human visual field maps, although this remains an active field of investigation, with more maps likely to be discovered. Finally, we Human visua l cortex comprises 4–6 billion neurons that are organ ized into more than a dozen distinct describe theories about the functional purpose and functional areas. These areas include the gray matter organizing principles of these maps. in the occi pital lobe and extend into the temporal and parietal lobes . The locations of these areas in the The Size and Location of Human Visual intact human cortex can be identified by measuring Cortex visual field maps. The neurons within these areas have a variety of different stimulus response proper- The entirety of human cortex occupies a surface area 2 ties. We descr ibe how to measure these visual field on the order of 1000 cm and ranges between 2 and maps, their locations, and their overall organization. 4 mm in thickness. Each cubic millimeter of cortex contains approximately 50 000 neurons so that neo- We then consider how information about patterns, objects, color s, and motion is analyzed and repre- cortex in the two hemispheres contain on the order of sented in these maps. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -
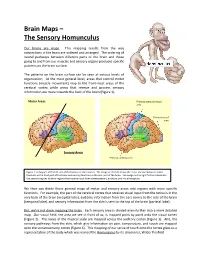
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Network Centrality in Patients with Acute Unilateral Open Globe Injury: a Voxel‑Wise Degree Centrality Study
MOLECULAR MEDICINE REPORTS 16: 8295-8300, 2017 Network centrality in patients with acute unilateral open globe injury: A voxel‑wise degree centrality study HUA WANG1, TING CHEN1, LEI YE2, QI-CHEN YANG3, RONG WEI2, YING ZHANG2, NAN JIANG2 and YI SHAO1,2 1Department of Ophthalmology, Xiangya Hospital, Central South University, Changsha, Hunan 410008; 2Department of Ophthalmology, The First Affiliated Hospital of Nanchang University, Jiangxi Province Clinical Ophthalmology Institute and Oculopathy Research Centre, Nanchang, Jiangxi 330006; 3Eye Institute of Xiamen University, Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, Xiamen, Fujian 361102, P.R. China Received January 12, 2017; Accepted August 1, 2017 DOI: 10.3892/mmr.2017.7635 Abstract. The present study aimed to investigate functional Introduction networks underlying brain-activity alterations in patients with acute unilateral open globe injury (OGI) and associations with Open globe injury (OGI) is a severe eye disease that frequently their clinical features using the voxel-wise degree centrality causes unilateral visual loss. Ocular trauma is a public health (DC) method. In total, 18 patients with acute OGI (16 males and problem in developing countries (1,2). A previous study indicated 2 females), and 18 healthy subjects (16 males and 2 females), that the annual prevalence of ocular trauma was 4.9 per 100,000 closely matched in age, sex and education, participated in the in the Western Sicily Mediterranean area, which investigated a present study. Each subject underwent a resting-state functional 5 year period from January 2001 to December 2005 (3). In addi- magnetic resonance imaging scan. The DC method was used tion, the incidence of OGI is increased in men compared with to assess local features of spontaneous brain activity. -

The Human Brain Hemisphere Controls the Left Side of the Body and the Left What Makes the Human Brain Unique Is Its Size
About the brain Cerebrum (also known as the The brain is made up of around 100 billion nerve cells - each one cerebral cortex or forebrain) is connected to another 10,000. This means that, in total, we The cerebrum is the largest part of the brain. It is split in to two have around 1,000 trillion connections in our brains. (This would ‘halves’ of roughly equal size called hemispheres. The two be written as 1,000,000,000,000,000). These are ultimately hemispheres, the left and right, are joined together by a bundle responsible for who we are. Our brains control the decisions we of nerve fibres called the corpus callosum. The right make, the way we learn, move, and how we feel. The human brain hemisphere controls the left side of the body and the left What makes the human brain unique is its size. Our brains have a hemisphere controls the right side of the body. The cerebrum is larger cerebral cortex, or cerebrum, relative to the rest of the The human brain is the centre of our nervous further divided in to four lobes: frontal, parietal, occipital, and brain than any other animal. (See the Cerebrum section of this temporal, which have different functions. system. It is the most complex organ in our fact sheet for further information.) This enables us to have abilities The frontal lobe body and is responsible for everything we do - such as complex language, problem-solving and self-control. The frontal lobe is located at the front of the brain.