Red-Eared Slider Turtle Risk Assessment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Competing Generic Concepts for Blanding's, Pacific and European
Zootaxa 2791: 41–53 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) Competing generic concepts for Blanding’s, Pacific and European pond turtles (Emydoidea, Actinemys and Emys)—Which is best? UWE FRITZ1,3, CHRISTIAN SCHMIDT1 & CARL H. ERNST2 1Museum of Zoology, Senckenberg Dresden, A. B. Meyer Building, D-01109 Dresden, Germany 2Division of Amphibians and Reptiles, MRC 162, Smithsonian Institution, P.O. Box 37012, Washington, D.C. 20013-7012, USA 3Corresponding author. E-mail: [email protected] Abstract We review competing taxonomic classifications and hypotheses for the phylogeny of emydine turtles. The formerly rec- ognized genus Clemmys sensu lato clearly is paraphyletic. Two of its former species, now Glyptemys insculpta and G. muhlenbergii, constitute a well-supported basal clade within the Emydinae. However, the phylogenetic position of the oth- er two species traditionally placed in Clemmys remains controversial. Mitochondrial data suggest a clade embracing Actinemys (formerly Clemmys) marmorata, Emydoidea and Emys and as its sister either another clade (Clemmys guttata + Terrapene) or Terrapene alone. In contrast, nuclear genomic data yield conflicting results, depending on which genes are used. Either Clemmys guttata is revealed as sister to ((Emydoidea + Emys) + Actinemys) + Terrapene or Clemmys gut- tata is sister to Actinemys marmorata and these two species together are the sister group of (Emydoidea + Emys); Terra- pene appears then as sister to (Actinemys marmorata + Clemmys guttata) + (Emydoidea + Emys). The contradictory branching patterns depending from the selected loci are suggestive of lineage sorting problems. Ignoring the unclear phy- logenetic position of Actinemys marmorata, one recently proposed classification scheme placed Actinemys marmorata, Emydoidea blandingii, Emys orbicularis, and Emys trinacris in one genus (Emys), while another classification scheme treats Actinemys, Emydoidea, and Emys as distinct genera. -

Redalyc.MORPHOLOGY and CONSERVATION of the MESOAMERICAN SLIDER (Trachemys Venusta, Emydidae) from the ATRATO RIVER BASIN, COLOMB
Acta Biológica Colombiana ISSN: 0120-548X [email protected] Universidad Nacional de Colombia Sede Bogotá Colombia CEBALLOS, CLAUDIA P.; BRAND, WILLIAM A. MORPHOLOGY AND CONSERVATION OF THE MESOAMERICAN SLIDER (Trachemys venusta, Emydidae) FROM THE ATRATO RIVER BASIN, COLOMBIA Acta Biológica Colombiana, vol. 19, núm. 3, septiembre-diciembre, 2014, pp. 483-488 Universidad Nacional de Colombia Sede Bogotá Bogotá, Colombia Available in: http://www.redalyc.org/articulo.oa?id=319031647014 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative SEDE BOGOTÁ ACTA BIOLÓGICA COLOMBIANA FACULTAD DE CIENCIAS DEPARTAMENTO DE BIOLOGÍA ARTÍCULO DE INVESTIGACIÓN MORPHOLOGY AND CONSERVATION OF THE MESOAMERICAN SLIDER (Trachemys venusta, EMYDIDAE) FROM THE ATRATO RIVER BASIN, COLOMBIA Morfología y conservación de la tortuga hicotea Mesoamericana (Trachemys venusta, Emydidae) del río Atrato, Colombia CLAUDIA P. CEBALLOS1, Ph. D.; WILLIAM A. BRAND2, Ecol. 1 Grupo Centauro. Escuela de Medicina Veterinaria, Facultad de Ciencias Agrarias, Universidad de Antioquia. Carrera 75 n.º 65-87, of. 47- 122, Medellín, Colombia. [email protected] 2 Corpouraba. Calle 92 n.º 98-39, Turbo, Antioquia, Colombia. [email protected] Author for correspondence: Claudia P. Ceballos, [email protected] Received 20th February 2014, first decision 14th May 2014, accepted 05th June 2014. Citation / Citar este artículo como: CEBALLOS CP, BRAND WA. Morphology and conservation of the mesoamerican slider (Trachemys venusta, Emydidae) from the Atrato River basin, Colombia. Acta biol. Colomb. 2014;19(3):483-488 ABSTRACT The phylogenetic relationships of the Mesoamerican Slider, Trachemys venusta, that inhabits the Atrato River basin of Colombia have been controversial as three different names have been proposed during the last 12 years: T. -

The Case of Deirocheline Turtles
bioRxiv preprint doi: https://doi.org/10.1101/556670; this version posted February 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Body coloration and mechanisms of colour production in Archelosauria: 2 The case of deirocheline turtles 3 Jindřich Brejcha1,2*†, José Vicente Bataller3, Zuzana Bosáková4, Jan Geryk5, 4 Martina Havlíková4, Karel Kleisner1, Petr Maršík6, Enrique Font7 5 1 Department of Philosophy and History of Science, Faculty of Science, Charles University, Viničná 7, Prague 6 2, 128 00, Czech Republic 7 2 Department of Zoology, Natural History Museum, National Museum, Václavské nám. 68, Prague 1, 110 00, 8 Czech Republic 9 3 Centro de Conservación de Especies Dulceacuícolas de la Comunidad Valenciana. VAERSA-Generalitat 10 Valenciana, El Palmar, València, 46012, Spain. 11 4 Department of Analytical Chemistry, Faculty of Science, Charles University, Hlavova 8, Prague 2, 128 43, 12 Czech Republic 13 5 Department of Biology and Medical Genetics, 2nd Faculty of Medicine, Charles University and University 14 Hospital Motol, V Úvalu 84, 150 06 Prague, Czech Republic 15 6 Department of Food Science, Faculty of Agrobiology, Food, and Natural Resources, Czech University of Life 16 Sciences, Kamýcká 129, Prague 6, 165 00, Czech Republic 17 7 Ethology Lab, Cavanilles Institute of Biodiversity and Evolutionary Biology, University of Valencia, C/ 18 Catedrátic José Beltrán Martinez 2, Paterna, València, 46980, Spain 19 Keywords: Chelonia, Trachemys scripta, Pseudemys concinna, nanostructure, pigments, chromatophores 20 21 Abstract 22 Animal body coloration is a complex trait resulting from the interplay of multiple colour-producing mechanisms. -
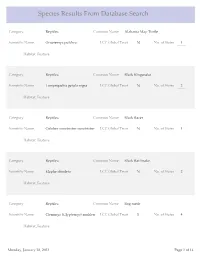
Species Results from Database Search
Species Results From Database Search Category Reptiles Common Name Alabama Map Turtle Scientific Name Graptemys pulchra LCC Global Trust N No. of States 1 Habitat_Feature Category Reptiles Common Name Black Kingsnake Scientific Name Lampropeltis getula nigra LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Black Racer Scientific Name Coluber constrictor constrictor LCC Global Trust N No. of States 1 Habitat_Feature Category Reptiles Common Name Black Rat Snake Scientific Name Elaphe obsoleta LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Bog turtle Scientific Name Clemmys (Glyptemys) muhlen LCC Global Trust Y No. of States 4 Habitat_Feature Monday, January 28, 2013 Page 1 of 14 Category Reptiles Common Name Broadhead Skink Scientific Name Eumeces laticeps LCC Global Trust N No. of States 5 Habitat_Feature Category Reptiles Common Name Coal Skink Scientific Name Eumeces anthracinus LCC Global Trust Y No. of States 8 Habitat_Feature Category Reptiles Common Name Common Five-lined Skink Scientific Name Eumeces fasciatus LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Common Map Turtle Scientific Name Graptemys geographica LCC Global Trust N No. of States 6 Habitat_Feature Category Reptiles Common Name Common Musk Turtle Scientific Name Sternotherus odoratus LCC Global Trust N No. of States 2 Habitat_Feature Monday, January 28, 2013 Page 2 of 14 Category Reptiles Common Name Common Ribbonsnake Scientific Name Thamnophis sauritus sauritus LCC Global Trust N No. of States 6 Habitat_Feature Category Reptiles Common Name Common Snapping Turtle Scientific Name Chelydra serpentina LCC Global Trust N No. of States 2 Habitat_Feature Category Reptiles Common Name Corn snake Scientific Name Elaphe guttata guttata LCC Global Trust N No. -

Movement and Habitat Use of Two Aquatic Turtles (Graptemys Geographica and Trachemys Scripta) in an Urban Landscape
Urban Ecosyst DOI 10.1007/s11252-008-0049-8 Movement and habitat use of two aquatic turtles (Graptemys geographica and Trachemys scripta) in an urban landscape Travis J. Ryan & Christopher A. Conner & Brooke A. Douthitt & Sean C. Sterrett & Carmen M. Salsbury # Springer Science + Business Media, LLC 2008 Abstract Our study focuses on the spatial ecology and seasonal habitat use of two aquatic turtles in order to understand the manner in which upland habitat use by humans shapes the aquatic activity, movement, and habitat selection of these species in an urban setting. We used radiotelemetry to follow 15 female Graptemys geographica (common map turtle) and each of ten male and female Trachemys scripta (red-eared slider) living in a man-made canal within a highly urbanized region of Indianapolis, IN, USA. During the active season (between May and September) of 2002, we located 33 of the 35 individuals a total of 934 times and determined the total range of activity, mean movement, and daily movement for each individuals. We also analyzed turtle locations relative to the upland habitat types (commercial, residential, river, road, woodlot, and open) surrounding the canal and determined that the turtles spent a disproportionate amount of time in woodland and commercial habitats and avoided the road-associated portions of the canal. We also located 21 of the turtles during hibernation (February 2003), and determined that an even greater proportion of individuals hibernated in woodland-bordered portions of the canal. Our results clearly indicate that turtle habitat selection is influenced by human activities; sound conservation and management of turtle populations in urban habitats will require the incorporation of spatial ecology and habitat use data. -
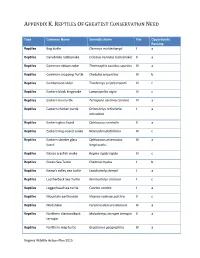
Reptiles of Greatest Conservation Need
APPENDIX K. REPTILES OF GREATEST CONSERVATION NEED Taxa Common Name Scientific Name Tier Opportunity Ranking Reptiles Bog turtle Clemmys muhlenbergii I a Reptiles Canebrake rattlesnake Crotalus horridus (canebrake) II a Reptiles Common ribbonsnake Thamnophis sauritus sauritus IV a Reptiles Common snapping Turtle Chelydra serpentina IV b Reptiles Cumberland slider Trachemys scripta troostii III c Reptiles Eastern black kingsnake Lampropeltis nigra III c Reptiles Eastern box turtle Terrapene carolina carolina III a Reptiles Eastern chicken turtle Deirochelys reticularia I a reticularia Reptiles Eastern glass lizard Ophisaurus ventralis II a Reptiles Eastern hog-nosed snake Heterodon platirhinos IV c Reptiles Eastern slender glass Ophisaurus attenuatus IV a lizard longicaudus Reptiles Glossy crayfish snake Regina rigida rigida III c Reptiles Green Sea Turtle Chelonia mydas I b Reptiles Kemp's ridley sea turtle Lepidochelys kempii I a Reptiles Leatherback Sea Turtle Dermochelys coriacea I c Reptiles Loggerhead sea turtle Caretta caretta I a Reptiles Mountain earthsnake Virginia valeriae pulchra II c Reptiles Mudsnake Farancia abacura abacura IV a Reptiles Northern diamondback Malaclemys terrapin terrapin II a terrapin Reptiles Northern map turtle Graptemys geographica IV a Virginia Wildlife Action Plan 2015 APPENDIX K. REPTILES OF GREATEST CONSERVATION NEED Reptiles Northern pinesnake Pituophis melanoleucus I a melanoleucus Reptiles Queen snake Regina septemvittata IV a Reptiles Rainbow snake Farancia erytrogramma IV a erytrogramma Reptiles -

( Malaclemys Terrapin Littoralis) at South Deer Island in Galveston Bay
DistrictCover.fm Page 1 Thursday, May 20, 2004 2:19 PM In cooperation with the U.S. Fish and Wildlife Service Occurrence of the Diamondback Terrapin (Malaclemys terrapin littoralis) at South Deer Island in Galveston Bay, Texas, April 2001–May 2002 Open-File Report 03–022 TEXAS Galveston Bay GULF OF EXICO M U.S. Department of the Interior U.S. Geological Survey Cover: Drawing of Diamondback terrapin by L.S. Coplin, U.S. Geological Survey. U.S. Department of the Interior U.S. Geological Survey Occurrence of the Diamondback Terrapin (Malaclemys terrapin littoralis) at South Deer Island in Galveston Bay, Texas, April 2001–May 2002 By Jennifer L. Hogan U.S. GEOLOGICAL SURVEY Open-File Report 03–022 In cooperation with the U.S. Fish and Wildlife Service Austin, Texas 2003 U.S. DEPARTMENT OF THE INTERIOR Gale A. Norton, Secretary U.S. GEOLOGICAL SURVEY Charles G. Groat, Director Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. For additional information write to District Chief U.S. Geological Survey 8027 Exchange Dr. Austin, TX 78754–4733 E-mail: [email protected] Copies of this report can be purchased from U.S. Geological Survey Information Services Box 25286 Denver, CO 80225–0286 E-mail: [email protected] ii CONTENTS Abstract ................................................................................................................................................................................ 1 Introduction ......................................................................................................................................................................... -

EMYDIDAE P Catalogue of American Amphibians and Reptiles
REPTILIA: TESTUDINES: EMYDIDAE P Catalogue of American Amphibians and Reptiles. Pseudemysj7oridana: Baur, 1893:223 (part). Pseudemys texana: Brimley, 1907:77 (part). Seidel, M.E. and M.J. Dreslik. 1996. Pseudemys concinna. Chrysemysfloridana: Di tmars, 1907:37 (part). Chrysemys texana: Hurter and Strecker, 1909:21 (part). Pseudemys concinna (LeConte) Pseudemys vioscana Brimley, 1928:66. Type-locality, "Lake River Cooter Des Allemands [St. John the Baptist Parrish], La." Holo- type, National Museum of Natural History (USNM) 79632, Testudo concinna Le Conte, 1830: 106. Type-locality, "... rivers dry adult male collected April 1927 by Percy Viosca Jr. of Georgia and Carolina, where the beds are rocky," not (examined by authors). "below Augusta on the Savannah, or Columbia on the Pseudemys elonae Brimley, 1928:67. Type-locality, "... pond Congaree," restricted to "vicinity of Columbia, South Caro- in Guilford County, North Carolina, not far from Elon lina" by Schmidt (1953: 101). Holotype, undesignated, see College, in the Cape Fear drainage ..." Holotype, USNM Comment. 79631, dry adult male collected October 1927 by D.W. Tesrudofloridana Le Conte, 1830: 100 (part). Type-locality, "... Rumbold and F.J. Hall (examined by authors). St. John's river of East Florida ..." Holotype, undesignated, see Comment. Emys (Tesrudo) concinna: Bonaparte, 1831 :355. Terrapene concinna: Bonaparte, 183 1 :370. Emys annulifera Gray, 183 1:32. Qpe-locality, not given, des- ignated as "Columbia [Richland County], South Carolina" by Schmidt (1953: 101). Holotype, undesignated, but Boulenger (1889:84) listed the probable type as a young preserved specimen in the British Museum of Natural His- tory (BMNH) from "North America." Clemmys concinna: Fitzinger, 1835: 124. -

In AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): Species in Red = Depleted to the Point They May Warrant Federal Endangered Species Act Listing
Southern and Midwestern Turtle Species Affected by Commercial Harvest (in AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): species in red = depleted to the point they may warrant federal Endangered Species Act listing Common snapping turtle (Chelydra serpentina) – AR, GA, IA, KY, MO, OH, OK, SC, TX Florida common snapping turtle (Chelydra serpentina osceola) - FL Southern painted turtle (Chrysemys dorsalis) – AR Western painted turtle (Chrysemys picta) – IA, MO, OH, OK Spotted turtle (Clemmys gutatta) - FL, GA, OH Florida chicken turtle (Deirochelys reticularia chrysea) – FL Western chicken turtle (Deirochelys reticularia miaria) – AR, FL, GA, KY, MO, OK, TN, TX Barbour’s map turtle (Graptemys barbouri) - FL, GA Cagle’s map turtle (Graptemys caglei) - TX Escambia map turtle (Graptemys ernsti) – FL Common map turtle (Graptemys geographica) – AR, GA, OH, OK Ouachita map turtle (Graptemys ouachitensis) – AR, GA, OH, OK, TX Sabine map turtle (Graptemys ouachitensis sabinensis) – TX False map turtle (Graptemys pseudogeographica) – MO, OK, TX Mississippi map turtle (Graptemys pseuogeographica kohnii) – AR, TX Alabama map turtle (Graptemys pulchra) – GA Texas map turtle (Graptemys versa) - TX Striped mud turtle (Kinosternon baurii) – FL, GA, SC Yellow mud turtle (Kinosternon flavescens) – OK, TX Common mud turtle (Kinosternon subrubrum) – AR, FL, GA, OK, TX Alligator snapping turtle (Macrochelys temminckii) – AR, FL, GA, LA, MO, TX Diamond-back terrapin (Malaclemys terrapin) – FL, GA, LA, SC, TX River cooter (Pseudemys concinna) – AR, FL, -

Three New Subspecies of Trachemys Venusta (Testudines: Emydidae) from Honduras, Northern Yucatán (Mexico), and Pacific Coastal Panama
Three New Subspecies of Trachemys venusta (Testudines: Emydidae) from Honduras, Northern Yucatán (Mexico), and Pacific Coastal Panama By William P. McCord1, Mehdi Joseph-Ouni2, Cris Hagen3, and Torsten Blanck4 1East Fishkill Animal Hospital, Hopewell Junction, NY, USA; 2EO Wildlife & Wilderness Conservation, Brooklyn NY, USA; 3Savannah River Ecology Laboratory, Aiken, SC, USA; 4Forstgartenstr 44, Deutschlandsberg, Austria Abstract. Upon examination of live and preserved specimens from across the species range, several unnamed distinct forms of Trachemys venusta (Gray, 1855) were recognized, leading to the description here of three biogeographically isolated, morphologically distinct subspecies: Trachemys venusta uhrigi ssp. nov., Trachemys venusta iversoni ssp. nov., and Trachemys venusta panamensis ssp. nov. Head and neck stripes, along with cara- pacial and plastral patterns are critical to identification in this group. Formal descriptions and diagnoses are given herein. Other Central American Trachemys are also discussed for comparison. Keywords: Turtle, emydid, Trachemys venusta uhrigi ssp. nov., Trachemys venusta iversoni ssp. nov., Trachemys venusta panamensis ssp. nov., Honduras, Mexico, Panama, Meso-America. Slider turtles of the genus Trachemys GRAY (1855) declared eight syntypes for his “Venus- Agassiz, 1857, range from north and east of like” Emys without designating a holotype. However, the Rio Grande in the United States, in 1873 he referred to only one syntype as “Emys through Mexico, Central America, the venusta”: stuffed specimen “e” (1845.8.5.26) in the West Indies, and South America as far as British Museum of Natural History, labeled “Charming northeastern Argentina. IVERSON (1985) resurrect- Emys” for its beautiful pattern — this reference led ed Trachemys from synonymy with Pseudemys (Gray, SMITH and SMITH (1979) to designate BMNH 1855). -

Report of Two Subspecies of an Alien Turtle, Trachemys Scripta Scripta and Trachemys Scripta Elegans (Testudines: Emydidae) Shar
Correspondence ISSN 2336-9744 (online) | ISSN 2337-0173 (print) The journal is available on line at www.ecol-mne.com Report of two subspecies of an alien turtle, Trachemys scripta scripta and Trachemys scripta elegans (Testudines: Emydidae) sharing the same habitat on the island of Zakynthos, Greece ALEKSANDAR UROŠEVI Ć University of Belgrade, Institute for Biological Research “Siniša Stankovi ć”, Bulevar despota Stefana 142, 11000 Belgrade, Serbia, E-mail: [email protected] Received 11 December 2014 │ Accepted 26 December 2014 │ Published online 28 December 2014. Introduction of alien aquatic turtles, especially the invasive red-eared slider, Trachemys scripta elegans (Wied-Neuwied 1839) has been noted as a global problem (Scalera 2006; Bringsøe 2006). In Greece only, introductions of the red-eared slider have already been published for several localities: Athens, Corfu, Crete, Kos and Zakynthos (Bruekers 1993; Bruekers et al . 2006; Zenetos et al . 2009). Until the EU banned the import and trade of the red-eared slider (Council Regulation No. 338/97), tens of millions of these turtles had been imported into Europe (Bringsøe 2006). Since the ban, the yellow-bellied slider Trachemys scripta scripta (Schoepff 1792) emerged in the European pet trade as one of the “substitute” species and subspecies (Adrados et al. 2002; Bringsøe 2006). Although it is sold in smaller quantities, and at a higher price, numbers of this subspecies found in the wild in Europe are increasing (Bringsøe 2006). The spread of T. s. scripta has been documented in Spain (Martínez Silvestre et al . 2006; Alarcos et al . 2010; Valdeón et al . 2010), Sweden, Finland (Bringsøe 2006) and Austria (Kleewein 2014). -

Blanding's Turtle
Blanding’s Turtle (Emydoidea blandingii): A Technical Conservation Assessment Prepared for the USDA Forest Service, Rocky Mountain Region, Species Conservation Project July 20, 2006 Justin D. Congdon, Ph.D.1 and Douglas A. Keinath2 1University of Georgia, Savannah River Ecology Laboratory, Drawer E, Aiken, SC 29802 2Wyoming Natural Diversity Database, University of Wyoming, P. O. Box 3381, Laramie, WY 82071 Peer Review Administered by Society for Conservation Biology Congdon, J.D. and D.A. Keinath. (2006, July 20). Blanding’s Turtle (Emydoidea blandingii): a technical conservation assessment. [Online]. USDA Forest Service, Rocky Mountain Region. Available: http://www.fs.fed.us/r2/ projects/scp/assessments/blandingsturtle.pdf [date of access]. ACKNOWLEDGMENTS We thank Dr. Jeff Lang for information about the Blanding’s turtle in Nebraska and Michael Pappas for information on breeding behavior of the Blanding’s turtle. Janet Hostetter, Owen Kinney, and Roy Nagle provided photographs. Nancy Dickson made comments on earlier drafts of the report. AUTHORS’ BIOGRAPHIES Justin Congdon is a Professor Emeritus at the University of Georgia’s Savannah River Ecology Laboratory (SREL) where he was a Senior Research Scientist until 2001. He holds adjunct positions at the University of Michigan and Arizona State University. Research focus at SREL was primarily on toxicology of reptiles, amphibians, and fish related to coal ash disposal. He has conducted long-term research on aspects of aging, ecology, and life histories of three species of turtles (painted turtles, Blanding’s turtles, and snapping turtles) on the University of Michigan’s Edwin S. George Reserve (31 years), the Sonoran mud turtles in the Chiricahua Mountains in southeastern Arizona (16 years), and on hatchling orientation and dispersal from nests and the composition of a seven species turtle community in the Weaver Dunes area in southeastern Minnesota (6 years).