Generation of a Radial-Like Glial Cell Line
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plp-Positive Progenitor Cells Give Rise to Bergmann Glia in the Cerebellum
Citation: Cell Death and Disease (2013) 4, e546; doi:10.1038/cddis.2013.74 OPEN & 2013 Macmillan Publishers Limited All rights reserved 2041-4889/13 www.nature.com/cddis Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum S-H Chung1, F Guo2, P Jiang1, DE Pleasure2,3 and W Deng*,1,3,4 NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre–Lox systems in vivo with two different mouse lines, the Plp-Cre-ERT2/Rosa26-EYFP and Olig2-Cre-ERT2/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2 þ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2 þ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic–ischemic injury, but the ability of NG2 þ cells to give rise to Bergmann glia and astrocytes remains unchanged. -

Neuregulin 1–Erbb2 Signaling Is Required for the Establishment of Radial Glia and Their Transformation Into Astrocytes in Cerebral Cortex
Neuregulin 1–erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex Ralf S. Schmid*, Barbara McGrath*, Bridget E. Berechid†, Becky Boyles*, Mark Marchionni‡, Nenad Sˇ estan†, and Eva S. Anton*§ *University of North Carolina Neuroscience Center and Department of Cell and Molecular Physiology, University of North Carolina School of Medicine, Chapel Hill, NC 27599; †Department of Neurobiology, Yale University School of Medicine, New Haven, CT 06510; and ‡CeNes Pharamceuticals, Inc., Norwood, MA 02062 Communicated by Pasko Rakic, Yale University School of Medicine, New Haven, CT, January 27, 2003 (received for review December 12, 2002) Radial glial cells and astrocytes function to support the construction mine whether NRG-1-mediated signaling is involved in radial and maintenance, respectively, of the cerebral cortex. However, the glial cell development and differentiation in the cerebral cortex. mechanisms that determine how radial glial cells are established, We show that NRG-1 signaling, involving erbB2, may act in maintained, and transformed into astrocytes in the cerebral cortex are concert with Notch signaling to exert a critical influence in the not well understood. Here, we show that neuregulin-1 (NRG-1) exerts establishment, maintenance, and appropriate transformation of a critical role in the establishment of radial glial cells. Radial glial cell radial glial cells in cerebral cortex. generation is significantly impaired in NRG mutants, and this defect can be rescued by exogenous NRG-1. Down-regulation of expression Materials and Methods and activity of erbB2, a member of the NRG-1 receptor complex, leads Clonal Analysis to Study NRG’s Role in the Initial Establishment of to the transformation of radial glial cells into astrocytes. -

The Interplay Between Neurons and Glia in Synapse Development And
Available online at www.sciencedirect.com ScienceDirect The interplay between neurons and glia in synapse development and plasticity Jeff A Stogsdill and Cagla Eroglu In the brain, the formation of complex neuronal networks and regulate distinct aspects of synaptic development and amenable to experience-dependent remodeling is complicated circuit connectivity. by the diversity of neurons and synapse types. The establishment of a functional brain depends not only on The intricate communication between neurons and glia neurons, but also non-neuronal glial cells. Glia are in and their cooperative roles in synapse formation are now continuous bi-directional communication with neurons to direct coming to light due in large part to advances in genetic the formation and refinement of synaptic connectivity. This and imaging tools. This article will examine the progress article reviews important findings, which uncovered cellular made in our understanding of the role of mammalian and molecular aspects of the neuron–glia cross-talk that perisynaptic glia (astrocytes and microglia) in synapse govern the formation and remodeling of synapses and circuits. development, maturation, and plasticity since the previ- In vivo evidence demonstrating the critical interplay between ous Current Opinion article [1]. An integration of past and neurons and glia will be the major focus. Additional attention new findings of glial control of synapse development and will be given to how aberrant communication between neurons plasticity is tabulated in Box 1. and glia may contribute to neural pathologies. Address Glia control the formation of synaptic circuits Department of Cell Biology, Duke University Medical Center, Durham, In the CNS, glial cells are in tight association with NC 27710, USA synapses in all brain regions [2]. -

Cerebellar Granule Cells in Culture
Proc. Nati. Acad. Sci. USA Vol. 83, pp. 4957-4961, July 1986 Neurobiology Cerebellar granule cells in culture: Monosynaptic connections with Purkinje cells and ionic currents (excitatory postsynaptic potential/patch-clamp) ToMoo HIRANO, YOSHIHIRo KUBO, AND MICHAEL M. WU Department of Neurobiology, Institute of Brain Research, School of Medicine, University of Tokyo, Tokyo, Japan Communicated by S. Hagiwara, March 6, 1986 ABSTRACT Electrophysiological properties of cerebellar tissue was dissociated by triturating with a fire-polished granule cells and synapses between granule and Purkinje cells Pasteur pipette in Ca-free Hanks' balanced salt solution were studied in dissociated cultures. Electrophysiological prop- containing 0.05% DNase and 12 mM MgSO4. The cells were erties of neurons and synapses in the mammalian central centrifuged at 150 x g at 40C and the pelleted cells were nervous system are best studied in dissociated cell cultures resuspended at a concentration of about 106 cells per ml in a because of good target cell visibility, control over the contents defined medium (9). One milliliter ofthe cell suspension from of the extracellular solution, and the feasibility of whole-cell newborn rats was plated first in a Petri dish (3.5 cm in patch electrode recording, which has been a powerful tech- diameter) containing several pieces ofheat-sterilized, poly(L- nique in analyzing biophysical properties of ionic channels in lysine)-coated coverslips, and then 1 ml of fetal cell suspen- small cells. We have applied this whole-cell recording technique sion was added. One-half of the culture medium was ex- to cultured cerebellar granule cells whose electrophysiological changed with fresh medium once a week. -

NEUROGENESIS in the ADULT BRAIN: New Strategies for Central Nervous System Diseases
7 Jan 2004 14:25 AR AR204-PA44-17.tex AR204-PA44-17.sgm LaTeX2e(2002/01/18) P1: GCE 10.1146/annurev.pharmtox.44.101802.121631 Annu. Rev. Pharmacol. Toxicol. 2004. 44:399–421 doi: 10.1146/annurev.pharmtox.44.101802.121631 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on August 28, 2003 NEUROGENESIS IN THE ADULT BRAIN: New Strategies for Central Nervous System Diseases ,1 ,2 D. Chichung Lie, Hongjun Song, Sophia A. Colamarino,1 Guo-li Ming,2 and Fred H. Gage1 1Laboratory of Genetics, The Salk Institute, La Jolla, California 92037; email: [email protected], [email protected], [email protected] 2Institute for Cell Engineering, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21287; email: [email protected], [email protected] Key Words adult neural stem cells, regeneration, recruitment, cell replacement, therapy ■ Abstract New cells are continuously generated from immature proliferating cells throughout adulthood in many organs, thereby contributing to the integrity of the tissue under physiological conditions and to repair following injury. In contrast, repair mechanisms in the adult central nervous system (CNS) have long been thought to be very limited. However, recent findings have clearly demonstrated that in restricted areas of the mammalian brain, new functional neurons are constantly generated from neural stem cells throughout life. Moreover, stem cells with the potential to give rise to new neurons reside in many different regions of the adult CNS. These findings raise the possibility that endogenous neural stem cells can be mobilized to replace dying neurons in neurodegenerative diseases. -

Endogenous Radial Glial Cells Support Regenerating Axons After Spinal
Cellular, molecular, and developmental neuroscience 871 Endogenous radial glial cells support regenerating axons after spinal cord transection Hiroshi Nomuraa, Howard Kimb, Andrea Mothea, Tasneem Zahirb, Iris Kulbatskia, Cindi M. Morsheadc, Molly S. Shoichetb and Charles H. Tatora During the development of central nervous system, radial NeuroReport 21:871–876 c 2010 Wolters Kluwer Health | glial cells support target-specific neuronal migration. Lippincott Williams & Wilkins. We recently reported that after implantation of chitosan NeuroReport 2010, 21:871–876 channels with complete spinal cord transection, the tissue bridging the spinal cord stumps contained axons and radial Keywords: channel implantation, chitosan, radial glial cell, spinal cord injury, spinal cord transection glial cells. The purpose of this study was to clarify the role of the radial glial cells in the tissue bridges. Chitosan aToronto Western Research Institute, Toronto Western Hospital, bDepartment of Chemical Engineering and Applied Chemistry and cDepartment of Surgery and channels were implanted in rats with thoracic spinal Institute of Medical Sciences, University of Toronto, Terrence Donnelly Centre for cord transection. After 14 weeks, all animals had tissue Cellular and Biomolecular Research, Toronto, Ontario, Canada bridges in the channels that contained many radial glial Correspondence to Charles H. Tator, MD, PhD, Toronto Western Research cells in longitudinal arrangement, some of which were Institute, Toronto Western Hospital, Room 12-423, McLaughlin Wing, 399 Bathurst Street, Toronto, Ontario M5T 2S8, Canada in contact with axons in the bridges. We suggest that Tel: + 1 416 603 5889; fax: + 1 416 603 5298; e-mail:[email protected] radial glial cells can guide regenerating axons across Received 25 May 2010 accepted 25 June 2010 the bridge in the channel after spinal cord transection. -

Extracellular Matrix: Functions in the Nervous System
Downloaded from http://cshperspectives.cshlp.org/ on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Extracellular Matrix: Functions in the Nervous System Claudia S. Barros, Santos J. Franco, and Ulrich Mu¨ller The Scripps Research Institute, Department of Cell Biology, Dorris Neuroscience Center, La Jolla, California 92037 Correspondence: [email protected] An astonishing number of extracellular matrix glycoproteins are expressed in dynamic patterns in the developing and adult nervous system. Neural stem cells, neurons, and glia express receptors that mediate interactions with specific extracellular matrix molecules. Functional studies in vitro and genetic studies in mice have provided evidence that the extra- cellular matrix affects virtually all aspects of nervous system development and function. Here we will summarize recent findings that have shed light on the specific functions of defined extracellular matrix molecules on such diverse processes as neural stem cell differentiation, neuronal migration, the formation of axonal tracts, and the maturation and function of syn- apses in the peripheral and central nervous system. xtracellular matrix (ECM) glycoproteins are NEURAL STEM CELL BEHAVIOR AND Ewidely expressed in the developing and adult NEURONAL MIGRATION nervous system. Tremendous progress has been made in defining the roles of specific ECM com- NSCs give rise to neurons and glia, and the ponents in controlling the behavior of neurons ECM provides a microenvironment that mod- and glia (Sanes 1989; Reichardt and Tomaselli ulates NSC behavior (Perris and Perissinotto 1991; Venstrom and Reichardt 1993; Milner 2000; Sobeih and Corfas 2002; Zimmermann and Campbell 2002; Nakamoto et al. 2004). and Dours-Zimmermann 2008). Radial glial Here, we will provide an overview of ECM func- cells (RGCs) of the developing central nervous tions in the nervous system, emphasizing recent system (CNS) are a well-studied class of NSCs findings that have shed light on the mechanisms (Fig. -

Control of Cerebellar Granule Cell Output by Sensory-Evoked Golgi Cell Inhibition
Control of cerebellar granule cell output by sensory-evoked Golgi cell inhibition Ian Duguid1,2,3, Tiago Branco1,4, Paul Chadderton5, Charlotte Arlt, Kate Powell6, and Michael Häusser3 Wolfson Institute for Biomedical Research and Department of Neuroscience, Physiology, and Pharmacology, University College London, London WC1E 6BT, United Kingdom Edited by Masao Ito, RIKEN Brain Science Institute, Wako, Japan, and approved September 1, 2015 (received for review May 25, 2015) Classical feed-forward inhibition involves an excitation–inhibition Results sequence that enhances the temporal precision of neuronal re- Sensory-Evoked Phasic and Spillover Golgi Cell Inhibition Precedes sponses by narrowing the window for synaptic integration. In Mossy Fiber Excitation in Granule Cells. Cerebellar granule cells the input layer of the cerebellum, feed-forward inhibition is thought receive direct phasic and indirect or “spillover” GABAergic in- to preserve the temporal fidelity of granule cell spikes during mossy put from Golgi cells (6, 16, 20, 21). To investigate the temporal fiber stimulation. Although this classical feed-forward inhibitory cir- dynamics of sensory-evoked inhibition in vivo, we recorded cuit has been demonstrated in vitro, the extent to which inhibition spontaneous and sensory-evoked excitatory (Vhold = −70 mV) shapes granule cell sensory responses in vivo remains unresolved. and inhibitory (Vhold = 0 mV) currents from the same granule Here we combined whole-cell patch-clamp recordings in vivo and cells in Crus II (Fig. 1 A–D). Granule cells were identified based dynamic clamp recordings in vitro to directly assess the impact of on their characteristic electrophysiological properties (Table S1), Golgi cell inhibition on sensory information transmission in the depth from the pial surface (>250 μm), and morphology (Fig. -

Differences in the Developmental Patterns of Three Microtubule-Associated Proteins in the Rat Cerebellum’
0270.8474/85/0504-0977$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 5, No. 4, pp. 977-991 Printed in U.S.A. April 1985 Differences in the Developmental Patterns of Three Microtubule-associated Proteins in the Rat Cerebellum’ ROBERT BERNHARDT,2 GERDA HUBER,3 AND ANDREW MATUS Friedrich Miescher-lnstitut, P. 0. Box 2543, CH-4002 Base/, Switzerland Abstract MAPS are potentially important physiological regulators of neuronal differentiation. The developmental distribution patternS of microtubule- It has become clear that microtubules of different tissues and cell associated proteins (MAPS) 1, 2, and 3 were studied using types are characterized by different MAPS. Thus, a category of high three monoclonal antibodies. lmmunochemical staining at the molecular weight MAPS, later identified as MAP2, is exclusively light and electron microscopic levels demonstrated the spe- expressed in adult neurons where it is most concentrated in den- cific localization of each MAP in different cellular and sub- drites (Matus et al., 1981, 1983; Caceres et al., 1983, 1984; Wiche cellular compartments. (i) MAPS, which is specifically asso- et al., 1983; Bernhardt and Matus, 1984; De Camilli et al., 1984). ciated with dendritic microtubules in the adult brain, is strictly This suggested the possibility that MAP2 could be specifically associated with growing dendrites from the onset of their involved in the formation of dendritic microtubules and hence could formation. (ii) MAP3, a recently described MAP of M, = play a significant role in the regulation of dendrite growth. This idea 180,000, which in the adult brain is associated with neurofi- found further support from immunohistochemical data of developing lament-rich axons and glial processes, is associated with brain (Bernhardt and Matus, 1982; Matus et al., 1983; Burgoyne and axons from the beginning of outgrowth. -

Neuron-Satellite Glial Cell Interactions in Sympathetic Nervous System Development
NEURON-SATELLITE GLIAL CELL INTERACTIONS IN SYMPATHETIC NERVOUS SYSTEM DEVELOPMENT by Erica D. Boehm A dissertation submitted to the Johns Hopkins University in conformity with the requirements for the degree of Doctor of Philosophy Baltimore, Maryland July 2020 © 2020 Erica Boehm All rights reserved. ABSTRACT Glial cells play crucial roles in maintaining the stability and structure of the nervous system. Satellite glial cells are a loosely defined population of glial cells that ensheathe neuronal cell bodies, dendrites, and synapses of the peripheral nervous system (Elfvin and Forsman 1978; Pannese 1981). Satellite glial cells are closely juxtaposed to peripheral neurons with only 20nm of space between their membranes (Dixon 1969). This close association suggests a tight coupling between the cells to allow for possible exchange of important nutrients, yet very little is known about satellite glial cell function and development. How neurons and glial cells co-develop to create this tightly knit unit remains undefined, as well as the functional consequences of disrupting these contacts. Satellite glial cells are derived from the same population of cells that give rise to peripheral neurons, but do not begin differentiation and proliferation until neurogenesis has been completed (Hall and Landis 1992). A key signaling pathway involved in glial specification is the Delta/Notch signaling pathway (Tsarovina et al. 2008). However, recent studies also implicate Notch signaling in the maturation of glia through non- canonical Notch ligands such as Delta/Notch-like EGF-related Receptor (DNER) (Eiraku et al. 2005). Interestingly, it has been reported that levels of DNER in sympathetic neurons may be dependent on the target-derived growth factor, nerve growth factor (NGF), and this signal is prominent in sympathetic neurons at the time in which satellite glial cells are developing (Deppmann et al. -

First Division, 44 Head Neural Induction and Maintenance, 45–46 Trunk
Index A3B5ϩ/PSA-NCAMϩ cells, 165 first division, 44 Ablation studies, 78 head neural induction and maintenance, 45–46 Acetylcholine (ACh), 295–296 trunk neural induction, 46–50 Acetylcholine esterase (AChE), 284 regional patterning, 50–57 Acetylcholine receptor (AChR) channels, 276 Antibodies that promote remyelination, 182 Acetylcholine receptor inducing activity (ARIA), 294 Apaf-1, 322–323 Acetylcholine receptors (AChRs), 273, 276, 278–281, 284, 290, 293, 309 Apoptosis-inducing factor (AIF), 319 distribution, 285 Apoptosis pathway, 353 types of, 269, 271 Apoptotic cell death, 318, 319; see also Programmed cell death Achaete-scute homologue ash1, 97 Ara-C (cytosine arabinoside), 205 Actin, retrograde flow of, 246 Architectonic maps, 396–397 Actin-binding protein filamin 1 (filamin-␣), 225 Astrocyte development Active zones (AZ), 270, 275, 279–280 in cerebellum, 208 Activin, 9, 10 in forebrain Adhesion, tactile, 377 pathways of, giving rise to different types of astrocytes, 207–208 Adhesion proteins, synaptic, 300–305 is not uniform across different regions of CNS, 199 Adhesive cell-surface signals, 251 in spinal cord, 208–209 Adrenoleukodystrophy, 173 Astrocyte genesis, interplay of multiple pathways contributes to, 216 Age-related alterations in neurogenesis, developmental mechanisms Astrocyte lineages underlying, 354–357 model of, 212 Age-related cytoarchitectural changes in nervous system, 350–351 in vitro, heterogeneity within, 211 Age-related molecular changes in nervous system, 351 Astrocyte precursor cells (APCs), 212–213 Age-related -
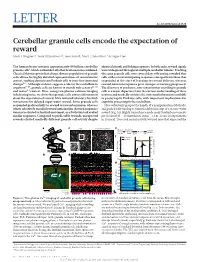
Cerebellar Granule Cells Encode the Expectation of Reward Mark J
LETTER doi:10.1038/nature21726 Cerebellar granule cells encode the expectation of reward Mark J. Wagner1*, Tony Hyun Kim1,2*, Joan Savall1, Mark J. Schnitzer1,3 & Liqun Luo1 The human brain contains approximately 60 billion cerebellar identical stimuli and licking responses. In both tasks, reward signals granule cells1, which outnumber all other brain neurons combined. were widespread throughout multiple cerebellar lobules. Tracking Classical theories posit that a large, diverse population of granule the same granule cells over several days of learning revealed that cells allows for highly detailed representations of sensorimotor cells with reward-anticipating responses emerged from those that context, enabling downstream Purkinje cells to sense fine contextual responded at the start of learning to reward delivery, whereas changes2–6. Although evidence suggests a role for the cerebellum in reward-omission responses grew stronger as learning progressed. cognition7–10, granule cells are known to encode only sensory11–13 The discovery of predictive, non-sensorimotor encoding in granule and motor14 context. Here, using two-photon calcium imaging cells is a major departure from the current understanding of these in behaving mice, we show that granule cells convey information neurons and markedly enriches the contextual information available about the expectation of reward. Mice initiated voluntary forelimb to postsynaptic Purkinje cells, with important implications for movements for delayed sugar-water reward. Some granule cells cognitive processing in the cerebellum. responded preferentially to reward or reward omission, whereas Mice voluntarily grasped the handle of a manipulandum (Methods) others selectively encoded reward anticipation. Reward responses and pushed it forward up to 8 mm for delayed receipt of a sucrose-water were not restricted to forelimb movement, as a Pavlovian task evoked reward (Fig.