Chinook Salmon Oncorhynchus Tshawytscha
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TRANS MOUNTAIN EXPANSION PROJECT: FISH-BEARING ATLAS Pipeline and Power Lines Supplemental Fisheries (British Columbia) Technical Report: Appendix B1
TRANS MOUNTAIN EXPANSION PROJECT: FISH-BEARING ATLAS Pipeline and Power Lines Supplemental Fisheries (British Columbia) Technical Report: Appendix B1 Prepared for: Trans Mountain Pipelines ULC Calgary, Alberta Submitted by: Triton Environmental Consultants Ltd. Kamloops, British Columbia December 2014 SREP-NEB-TERA-00030 FISH-BEARING ATLAS Pipeline and Power Lines PREPARED AS APPENDIX B1 OF THE SUPPLEMENTAL FISHERIES (BRITISH COLUMBIA) TECHNICAL DISCIPLINE REPORT GLOSSARY AND KEY OF TERMINOLOGY, ABBREVIATIONS, AND SYMBOLS USED IN THE FISH-BEARING ATLAS FOR PIPELINE AND POWER LINES Channel Morphology Pattern The path of a channel in relation to a straight line. A qualitative method of assessing sinuosity. Confinement The ability of the channel to migrate laterally on a valley flat between surrounding slopes. Bank Shape The shape or form of the identified channel bank described when the observer is facing downstream. Habitat Unit Description of the morphological unit observed within the section investigated. Gradient The slope or rate of vertical drop per unit of length, of the channel bed. Main Stem The name of and distance to the nearest watercourse known to be fish-bearing (FB) habitat, as measured from the approximate proposed crossing location. Wetted Width The width of the water surface at the time of survey; measured at right angles to the direction of flow. Channel Width The distance between the ordinary high water mark of both right and left banks. Bank Height The height measured from the channel bottom at the watercourse’s deepest point at the transect to the bank’s break in slope at its top, such that the grade beyond the break is flatter than 1:3 (rise:run) at any point for a minimum of 15 m measured perpendicularly to the bank. -

Habitat-Based Methods to Estimate Spawner Capacity for Chinook
C S A S S C C S Canadian Science Advisory Secretariat Secrétariat canadien de consultation scientifique Research Document 2002/114 Document de recherche 2002/114 Not to be cited without Ne pas citer sans permission of the authors * autorisation des auteurs * Habitat-based methods to estimate Méthodes axées sur l’habitat pour estimer spawner capacity for chinook salmon in la capacité d’accueil de saumons the Fraser River watershed quinnats géniteurs dans le réseau fluvial du fleuve Fraser C. K. Parken1 , J. R. Irvine1, R. E. Bailey2, I. V. Williams3 1Fisheries and Oceans Canada Science Branch, Stock Assessment Division Pacific Biological Station Nanaimo, B.C. V9T 6N7 2Fisheries and Oceans Canada BC Interior, Resource Management 1278 Dalhousie Drive Kamloops, B.C. V2B 6G3 3I.V. Williams Consulting Ltd. 3565 Planta Rd. Nanaimo, B.C. V9T 1M1 * This series documents the scientific basis for the * La présente série documente les bases scientifiques evaluation of fisheries resources in Canada. As such, des évaluations des ressources halieutiques du Canada. it addresses the issues of the day in the time frames Elle traite des problèmes courants selon les échéanciers required and the documents it contains are not dictés. Les documents qu’elle contient ne doivent pas intended as definitive statements on the subjects être considérés comme des énoncés définitifs sur les addressed but rather as progress reports on ongoing sujets traités, mais plutôt comme des rapports d’étape investigations. sur les études en cours. Research documents are produced in the official Les documents de recherche sont publiés dans la language in which they are provided to the langue officielle utilisée dans le manuscrit envoyé au Secretariat. -

Assessment of Non-Natural Coho Barriers in the North Thompson Watershed
Secwepemc Fisheries Commission Third Quarter Report 2002-2003 Assessment of Non-Natural Coho Barriers in the North Thompson Watershed Forest Investment Account Project 4205014 Secwepemc Fisheries Commission Third Quarter Report 2002-2003 Assessment of Non-Natural Coho Barriers in the North Thompson Watershed Forest Investment Account Project 4205014 Prepared For: Tolko Industries Ltd. Louis Creek Division C/O Michael Bragg, Divisional Forester Site 10, Comp. 10, RR #3 Kamloops, BC V2C 5K1 Prepared By: Shawn Clough Secwepemc Fisheries Commission #274-A Halston Connector Road Kamloops, B.C. V2H 1J9 Phone: (250) 828-2178 Fax: (250) 828-2756 January 2004 Secwepemc Fisheries Commission ACKNOWLEDGEMENTS The Secwepemc Fisheries Commission would like to thank Michael Bragg and Tolko Industries Ltd. for their support in completing this project. Tolko Industries, through the Forest Investment Account (FIA), provided the funding for this assessment, and allowed the project to be completed on the most critical coho producing streams both within and outside Tolko’s timber license operating boundaries. In addition, we would like to acknowledge the assistance and support of the North Thompson Indian Band. The Simpcw people have a vested interest in ensuring fish, and in particular salmon species, have quality spawning and rearing habitat available. Their dedication to this cause initiated the project. Finally, we would like to thank Cascadia Natural Resource Consultants Ltd. for their high quality mapping services, without which, some of these culverts would not have been located! Fennell Creek Log Culvert Barrier Assessment of Non-Natural Coho Barriers in the North Thompson Page I Secwepemc Fisheries Commission EXECUTIVE SUMMARY Interior Fraser coho salmon (Oncorhynchus kisutch) have been recommended for endangered classification under the Species at Risk Act. -

The Cariboo and Monashee Ranges of British Columbia: an Alpinist’S Guide
1 THE CARIBOO AND MONASHEE RANGES OF BRITISH COLUMBIA: AN ALPINIST’S GUIDE by EARLE R. WHIPPLE Even today, British Columbia is still a wilderness of mountains, valleys, glaciers, forest and plateau. The Columbia Mountains (Interior Ranges; which include the Cariboo and Monashee Ranges) lie within British Columbia, west of the Canadian Rockies and the southern Alberta-British Columbia border. This guide describes the access and mountaineering in these two ranges. Aside from parts of the Coast Range and the northern Rockies, the Cariboo and Monashee Ranges are the most isolated in B.C. However, if one listens to the helicopters from the lodges in these ranges, when camped there, one may question this. Large, active glaciers (now in retreat) with spectacular icefalls exist in the mountains of the western part of the Halvorson Group, the northern Wells Gray Group, the Premier Ranges, the Dominion Group and northern Scrip Range; there is climbing on rock, snow and ice, and routes for those climbers wishing easy, relaxing climbing in beautiful scenery. Good rock climbing on gneiss is in the southern Gold Range and Mt. Begbie in the north. There are also locales offering fine hiking on trails or alpine meadows (Halvorson Group, southern Wells Gray Group, southern Scrip Range, and the Shuswap Group), and backpacking traverses have been worked out through the Halvorson and Dominion Groups, the Scrip Range and the Gold Range. Beautiful lake districts exist in the northern Cariboos, and the Monashees. The area covered by this book starts northwest of the town of McBride, on Highway 16, southeast of Prince George, and extends south to near the border with the U.S.A., staying within the great bend of the Fraser River, and then west of Canoe Reach (lake; formerly Canoe River) and just west of the lower Columbia River south of its great bend. -
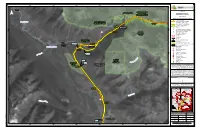
Page 1 !( !( !( !( !. !. !. !. !. !. !. !. "/ "/ WT-1 WT-1088 WT-1121 WT-2 WT
0 0 0 330000 335000 340000 345000 350000 355000 0 0 0 0 0 8 8 8 8 5 5 S h e r e FIGURE A2 M o u n t R o b s o n MOUNT ROBSON COR RIDOR WETLAND DELINEATION - ¯ PROTECTED AREA HARGREAVES TO DARFIELD SHEET 1 OF 10 TRANS MOUNTAIN EXPANSION PROJECT !. Kilometre Post (KP) Fraser River Trans Mountain Pipeline (TMPL) MOUNT ROBSON !. PROTECTED AREA !. Reference Kilometre Post (RK) KP 460 Trans Mountain Expansion Project Proposed Pipeline Corridor RK 490 "/ Proposed Power Line !. Hargreaves *# Terminal 0 0 0 0 0 0 Pump Station (Pump Additions, Station 5 Trap Site 5 "/ 7 7 Modifications and/or Scraper Facilities) 8 8 5 5 "/ New Pump Station (Proposed) 16 !. OP KP 470 MOUNT "/ Pump Station (Reactivated) ROBSON "/ Existing Pump Station PARK (!1 Highway Paved Road !. Railway REARGUARD Village / Hamlet / Community FALLS PARK City / Town Indian Reserve / Métis Settlement T ê t e WT-1088 National Park / Provincial Park / Protected Area J a u n e RK 500!. "/ Municipal / District Boundary C a c h e Mclennan River Rearguard Wetland Classifications (! !( Alkali Marsh !. (! !( Broad-leaf Treed Swamp KP 480 Deep Marsh 0 0 0 0 0 0 !( Mixedwood Treed Swamp 0 0 7 7 8 ek 8 !( Needle-leaf Treed Swamp 5 re WT-1121 5 ete C !( Non-Woody Fen T Open Water Pond !( Seasonal Emergent Marsh k e !( Shrubby Fen re MOUNT C !( Shrubby Swamp WT-1 n TERRY o !( Treed Bog M rath JACKMAN WT-2 FOX PARK a !( Treed Fen This document is provided by Kinder Morgan Canada Inc. -

For Fraser River Chinook Salmon Conservation) Pour Le Saumon Quinnat Du Fraser
C S A S S C C S Canadian Science Advisory Secretariat Secrétariat canadien de consultation scientifique Research Document 2002/085 Document de recherche 2002/085 Not to be cited without Ne pas citer sans permission of the authors * autorisation des auteurs * A discussion paper on possible new Document de travail sur les nouveaux stock groupings (Conservation Units) agrégats possibles de stocks (unités de for Fraser River chinook salmon conservation) pour le saumon quinnat du Fraser J. R. Candy1, J. R. Irvine1, C. K. Parken1, S. L. Lemke2, R. E. Bailey2, M. Wetklo1 and K. Jonsen1 1 Fisheries and Oceans Canada Science Branch, Pacific Biological Station Nanaimo, B.C. V9T 6N7 2Fisheries and Oceans Canada B.C. Interior, Resource Management 1278 Dalhousie Drive, Kamloops, B.C. V2B 6G3 * This series documents the scientific basis for the * La présente série documente les bases scientifiques evaluation of fisheries resources in Canada. As such, des évaluations des ressources halieutiques du Canada. it addresses the issues of the day in the time frames Elle traite des problèmes courants selon les échéanciers required and the documents it contains are not dictés. Les documents qu’elle contient ne doivent pas intended as definitive statements on the subjects être considérés comme des énoncés définitifs sur les addressed but rather as progress reports on ongoing sujets traités, mais plutôt comme des rapports d’étape investigations. sur les études en cours. Research documents are produced in the official Les documents de recherche sont publiés dans la language in which they are provided to the langue officielle utilisée dans le manuscrit envoyé au Secretariat. -

Valemount & Area Environmental Background
Valemount & Area Environmental Background Report Prepared to provide background information to support the development of the Valemount and Area Integrated Land Use Development Plan Prepared By : Beryl Nesbit, Planning Biologist MSRM Rhonda Thibeault, Land and Resource Analyst MSRM Gordon Borgstrom, Manager of Regional Planning Specialists & Tourism Land Use, MSRM FOREWORD The area surrounding the Village of Valemount is poised for significant change. Although the landscape of the area has already been altered by settlement, major infrastructure corridors and natural resource extraction activities, it seems very likely that major resort developments will significantly increase the population and ecological “footprint” of the community in the next two decades. The purpose of this paper is two-fold: first, to identify and discuss the existing environmental information knowledge base for the area – specifically, known sensitive and environmentally important species, lands, waters, and ecological parameters for the region; second, recognizing that the area is likely to see significant population and settlement growth in the near future, the paper makes recommendations on appropriate actions for the Village, Regional District, and Provincial governments to consider in order to help preserve important environmental attributes in the area. This paper has been prepared by Ministry of Sustainable Resource Management (MSRM) staff as a technical background paper to support the completion and preparation of the Valemount and Area Integrated Land Use Development Plan. MSRM is appreciative of the support of Ministry of Water, Land, and Air Protection Environmental Stewardship staff that have reviewed and commented on this paper. 1 1 Canoe Mountain as viewed from Highway 5 south of Valemount 2 Table of Contents Foreword pg.2 1.0 Purpose and Background pg.6 2.0 Introduction pg.7 3.0 Fish and Wildlife Species in the Planning Area pg.9 3.1 Fish and Wildlife Overview pg.9 3.2 Fish pg. -
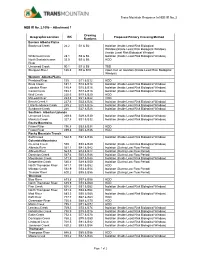
Attachment 1 Geographic Location RK Drawing Numbers Proposed Prima
Trans Mountain Response to NEB IR No. 2 NEB IR No. 2.105b – Attachment 1 Drawing Geographic Location RK Proposed Primary Crossing Method Numbers Eastern Alberta Plains Blackmud Creek 24.2 B1 & B2 Isolation (Inside Least Risk Biological Window)(Inside Least Risk Biological Window) (Inside Least Risk Biological Window) Whitemud Creek 28.1 B3 & B4 Isolation (Inside Least Risk Biological Window) North Saskatchewan 33.5 B5 & B6 HDD River Unnamed Creek 90.1 B7 & B8 TBD Sturgeon River 126.8 B9 & B10 Open Cut or Isolation (Inside Least Risk Biological Window) Western Alberta Plains Pembina River 135 B11 & B12 HDD Brule Creek 181.1 B13 & B14 Isolation (Inside Least Risk Biological Window) Lobstick River 185.4 B15 & B16 Isolation (Inside Least Risk Biological Window) Carrot Creek 193.1 B17 & B18 Isolation (Inside Least Risk Biological Window) Wolf Creek 220.6 B19 & B20 HDD McLeod River 223.8 B21 & B22 HDD Bench Creek 1 227.6 B23 & B24 Isolation (Inside Least Risk Biological Window) Little Sundance Creek 245.2 B25 & B26 Isolation (Inside Least Risk Biological Window) Sundance Creek 248 B27 & B28 Isolation (Inside Least Risk Biological Window) Southern Albertan Uplands Unnamed Creek 269.6 B29 & B30 Isolation (Inside Least Risk Biological Window) Maskuta Creek 327.8 B31 & B32 Isolation (Inside Least Risk Biological Window) Rocky Mountains Fraser River 496.8 B33 & B34 HDD Fraser River 499.8 B35 & B36 HDD Rocky Mountain Trench Swift Creek 522.5 B37 & B38 Isolation (Inside Least Risk Biological Window) Columbia Mountains Clemina Creek 559 B39 & B40 Isolation -

Op Op Op Op Op Op Op Op
! F ra s ALBERTA er R BRITISH Isaac iv Lake er COLUMBIA Bowron Lake Provincial Park 120°30'0"W 120°0'0"W 119°30'0"W 119°0'0"W 118°30'0"W ! Dunster 16 Mount Robson Lanezi OP Provincial Park Lake Mount Robson ! 53°0'0"N ¯ Hargreaves Trap Site Ghost "/ Lake KP 450 Rearguard !. JASPER ! "/ !. NATIONAL 53°0'0"N Tête Jaune Cache Mitchell RK 500 PARK Lake Cariboo Mountains Moose Lake Provincial Park OP16 !.KP 400 OP5 ! VALEMOUNT !. KP 500 ! F Cedarside r a s e r ! R iv er ! ! Albreda "/ N ! Albreda o Quesnel r !. th Lake T h RK 550 o m p so Hobson n Lake R ive 52°30'0"N r 52°30'0"N r Horsefly e iv Lake R Azure KP 550 fly !. orse Lake H Angus ! Chappel Horne "/ Lake ! Crooked OP5 Lake Clearwater Mcdougall Tisdall Lake Lake Lake !. RK 600 Kostal Lake Wells Gray Provincial Blue River Murtle Kinbasket Park Lake "/! Blue River Lake OP23 C C o 52°0'0"N l l e KP 600 !. u a m r w b i Schoolhouse Lake a a t 52°0'0"N R Provincial Park e r i v R e Mahood iv r e Lake r "/ ! Finn Eagle Canim Lake ! Tumtum Creek Mahood Falls Lake !. ! ! Canim Lake ! RK 650 ! ! Avola ! Taweel KP 650 Clearwater !. "/ ! ! Upper Seymour Provincial KP 700 McMurphy ! Park !. River Provincial Deka Lake Park ! OP5 Blackpool ! ! ! RK 700 Blackpool Birch !. ! "/ Island Vavenby Roe Lake! Sheridan 51°30'0"N 24 Lake ! OP OP23 Bridge RK 750 !. -

4.0 CORRIDOR and FACILITY SITE SELECTION the Project Includes Further Looping of the Existing 1,150 Km TMPL System from Edmonton to Burnaby in Operation Since 1953
Trans Mountain Pipeline ULC Volume 5B: ESA – Socio-Economic Trans Mountain Expansion Project Section 4.0: Corridor and Facility Site Selection 4.0 CORRIDOR AND FACILITY SITE SELECTION The Project includes further looping of the existing 1,150 km TMPL system from Edmonton to Burnaby in operation since 1953. The 987 km of pipeline that will be looped as part of TMEP traverses a wide range of landforms from flat farmland to mountainous terrain. Land use varies from densely populated urban areas around Edmonton, Vancouver and elsewhere to sparsely populated rural agricultural and forested Crown lands. The pipeline segments to be constructed as part of the Project will also potentially cross over 500 rivers and streams, 8 provincial parks and 13 Indian Reserves (IRs). An overview of the general routing objectives/criteria and proposed pipeline corridor is provided in Section 4.2 of Volume 2. A more detailed description of the pipeline corridor and selection process is provided in Section 2.8 of Volume 4A. This section provides an overview of the selection process for the proposed pipeline corridor, including a discussion of how environmental, socio-economic, Aboriginal engagement, stakeholder consultation and other factors influenced pipeline corridor selection. While the proposed pipeline will generally require a construction right-of-way of 45 m, a 150 m corridor was selected to define the boundaries of the environmental resource surveys, landowner contacts and other survey needs. This section also describes the site selection for permanent facilities such as terminals, pump stations (including access roads and power lines) and mainline block valves, as well as the site selection process for temporary facilities used during construction, such as staging and stockpile sites, equipment storage sites, construction office sites, construction work camps, work areas for trenchless watercourse crossings, temporary access roads, borrow pits and log decks. -
Fisheries (British Columbia) Technical Report for the Trans Mountain Pipeline Ulc Trans Mountain Expansion Project
FISHERIES (BRITISH COLUMBIA) TECHNICAL REPORT FOR THE TRANS MOUNTAIN PIPELINE ULC TRANS MOUNTAIN EXPANSION PROJECT December 2013 REP-NEB-TERA-00006 Prepared for: Prepared by: Trans Mountain Pipeline ULC Kinder Morgan Canada Inc. Triton Environmental Consultants Suite 2700, 300 – 5th Avenue S.W. 1326 McGill Road Calgary, Alberta T2P 5J2 Kamloops, BC V2C 6N6 Ph: 403-514-6400 Ph: 250-851-0023 Volume 5C, ESA - Trans Mountain Pipeline ULC Environmental Technical Reports Trans Mountain Expansion Project Fisheries (British Columbia) Technical Report ACKNOWLEDGEMENTS Trans Mountain Pipeline ULC would like to acknowledge Chief and Council, the Lands Department, Administration and members of the following communities: Lheidli T’enneh; Aseniwuche Winewak Nation; Simpcw First Nation; Tk'emlúps te Secwe̓pemc; Lower Nicola Indian Band; Nicola Tribal Association on behalf of Nooaitch Indian Band, Nicomen Indian and Shackan Indian Band; Yale First Nation; Chawathil First Nation; Shxw’ow’hamel First Nation; Cheam First Nation; Seabird Island Band; Popkum First Nation; Scowlitz First Nation; Le’qa:mel First Nation; and Kwantlen First Nation. All of their time, effort, commitment and participation is much appreciated and was fundamental to the success of the fisheries field surveys for the proposed Trans Mountain Expansion Project. December 2013 REP-NEB-TERA-00006 Page i Volume 5C - Trans Mountain Pipeline ULC Biophysical Technical Reports Trans Mountain Expansion Project Fisheries (British Columbia) Technical Report EXECUTIVE SUMMARY Fish and fish habitat investigations were carried out to satisfy application and regulatory requirements in support of an environmental and socio-economic assessment for the Trans Mountain Expansion Project (the Project). The approximately 674.4 km proposed pipeline corridor (BC portion) will pass through 13 watersheds and comprises four pipeline segments (Hargreaves to Darfield, Black Pines to Hope, Hope to Burnaby and Burnaby to Westridge) and two power lines (Black Pines power line and Kingsvale power line). -

Canadian and American Notes
432 Canadian and American No tes. CANADIAN AND AMERICAN NOTES. LEVELLINGUP MOUNT WHITNEY. THE official height of Mt. Whitney, the highest point within the United States outside Alaska, has been changed frequently since th e summit was first discovered in 1864 by Clarence King and Richard Cotter of the California State Geological Survey. These early sur veyors dispelled the rather vague notion of a range of mountains 17,000 ft. high standing on th e E. side of th e Sierra Nevada range, and gauged the new-found peak to be some 350 ft. higher than Mt. Tyndall, th e one upon which they stood I n July 1864, Clarence King again attempted to reach the summit of Mt. Whitney, but failed by 300 or 400 ft. According to the most reliable calculations King had reached a height of 14,740 ft., so th at the summit of th e mountain was estimated to be about 15,000 ft. The Times Atlas still persists in giving Mt. Whitney thi s altitude despite the fact th at later surveys have shown the summit to be some 500 ft . lower, and until last year the height of 14,501 ft. was the generally accepted one. Being exactly half the height of Mt.Everest tbis was a convenient figure to remember, but the U.S. Coast and Geodetic Survey spent part of th e summer of 1928 in depriving Mt. Whitney of this touch of sentiment. The height of Mt. Whitney has changed again, and again for the worse, but thi s tim e th e drop is not a serious matter.