Regarding Issues of the Safe Supply Options for Opioids in the “Risk Mitigation in the Context of Dual Public Health Emergencies” Guideline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regulatory Information Sheet
Regulatory Information Sheet List of Permanently Listed Color Additives Subject to U.S. Certification* Color 21 CFR References CAS Color Common Name Index Medical Number Food Drug Cosmetic Number Devices D&C Black No. 2 Carbon Black 77266 1333-86-4 -- -- 74.2052 -- D&C Black No. 3 Bone Black 77267 8021-99-6 -- -- 74.2053 -- FD&C Blue No. 1 Brilliant Blue FCF 42090 2650-18-2 74.101 74.1101 74.2101 -- FD&C Blue No. 2 Indigotine 73015 860-22-0 74.102 74.1102 -- 74.3102 D&C Blue No. 4 Alphazurine FG 42090 6371-85-3 -- 74.1104 74.2104 -- D&C Blue No. 6 Indigo 73000 482-89-3 -- -- -- 74.3106 D&C Blue No. 9 Indanthrene Blue 69825 130-20-1 -- 74.1109 -- -- D&C Brown No. 1 Resorcin Brown 20170 1320-07-6 -- -- 74.2151 -- FD&C Green No. 3 Fast Green FCF 42053 2353-45-9 74.203 74.1203 74.2203 -- D&C Green No. 5 Alizarin Cyanine Green F 61570 4403-90-1 -- 74.1205 74.2205 -- D&C Green No. 6 Quinizarine Green SS 61565 128-80-3 -- 74.1206 74.2206 74.3206 D&C Green No. 8 Pyranine Concentrated 59040 6358-69-6 -- 74.1208 74.2208 -- Orange B -- 19235 -- 74.250 -- -- -- D&C Orange No. 4 Orange II 15510 633-96-5 -- 74.1254 74.2254 -- D&C Orange No. 5 Dibromofluorescein 45370:1 596-03-2 -- 74.1255 74.2255 -- D&C Orange No. 10 Diiodofluorescein 45425:1 3329-19-9 -- 74.1260 74.2260 -- D&C Orange No. -

Evaluating Color Additives and Flavors Intended for Oral Drug Products Submitted Or Referenced in Inds and Ndas
MANUAL OF POLICIES AND PROCEDURES CENTER FOR DRUG EVALUATION AND RESEARCH MAPP 5021.2 POLICY AND PROCEDURES OFFICE OF PHARMACEUTICAL QUALITY Evaluating Color Additives and Flavors Intended for Oral Drug Products Submitted or Referenced in INDs and NDAs Table of Contents PURPOSE ..............................................................................1 BACKGROUND ...................................................................2 POLICY .................................................................................4 RESPONSIBILITIES ...........................................................5 PROCEDURES .....................................................................7 EFFECTIVE DATE ..............................................................9 CHANGE CONTROL TABLE ............................................9 Attachment 1: Listing of Color Additives Subject to Certification (21 CFR Part 74, Subpart B, Drugs) .............10 Attachment 2: Listing of Color Additives Exempt From Certification (Subpart B, Drugs) ........................................12 PURPOSE This MAPP describes the policies, procedures, and responsibilities in the Office of Pharmaceutical Quality (OPQ), in collaboration with the Office of New Drugs (OND), for assessing a color additive1 or a flavor present as an excipient in an oral drug product submitted as part of an investigational new drug application (IND), new drug application (NDA), NDA supplement, or an amendment to one of these submissions, or referenced in a Type IV drug master file (DMF).2,3 Specifically, -

Analysis of Artificial Colorants in Various Food Samples Using Monolithic Silica Columns and LC-MS by Stephan Altmaier, Merck Millipore, Frankfurter Str
31 Analysis of Artificial Colorants in Various Food Samples using Monolithic Silica Columns and LC-MS by Stephan Altmaier, Merck Millipore, Frankfurter Str. 250, 64293 Darmstadt, Germany This work describes a simple and sensitive high performance liquid chromatography method with UV or mass spectrometry detection for the analysis of artificial colorants from dye classes such as azo or chinophthalone in various food samples. After a short sample preparation procedure all samples were separated on C18 reversed phase monolithic silica columns via a gradient elution profile and directly transferred to UV or MS for the analysis of the main components. This setup enabled the identification of dyes in real life samples such as beverages or sweets within very short analysis times and with a minimised sample preparation step. In the nineteenth century chemicals such as azo compounds (see Tables 1 and 2 and by an organism very easily. mercury sulphide, lead oxide, copper salts or Figure 1). They are utilised as a single Most of the current artificial colorants can now fuchsine were utilised to artificially colour colouring ingredient or as a mixture with be replaced by natural dyes very easily. food such as cheese, confectionary, pickles other colorants in a wide variety of foods and Nevertheless, for economic reasons they are [1] or wine [2]. In the end of that century the beverages. All listed dyes are nontoxic and still used to improve the attractiveness of discovery of many synthetic organic food water soluble and can therefore be excreted sweets or soft drinks towards children or of colorants allowed for more brilliant colours than traditional natural dyes. -

List of Colorants in Cosmetic Products
List of Colorants in Cosmetic Products This rule has been translated into English according to the original Chinese version. If there is any inconsistency or ambiguity between these two versions, the Chinese version shall prevail. Explanation of Colorants Classification: Class 1: Colorants allowed in all cosmetic products Class 2: Colorants allowed in all cosmetic products except those intended to be applied in the vicinity of the eyes Class 3: Colorants allowed exclusively in cosmetic products intended not to come into contact with the mucous membranes Class 4: Colorants allowed exclusively in cosmetic products intended to come into contact only briefly with the skin Colour Index Number/ Scope of Number Alias Name Restriction Ingredient Name Application 1 CI 10006 Pigment Green 8 4 Acid Green 1 3 Using in hair dye 2 CI 10020 Ext. D&C Green No. 1 products is forbidden. Naphthol Green B Acid Yellow 1 2 3 CI 10316 Ext. D&C Yellow No. 7 Naphthol Yellow S Pigment Yellow 1 3 4 CI 11680 Ext. D&C Yellow No. 5 Hansa Yellow G 5 CI 11710 Pigment Yellow 3 3 Pigment Orange 1 4 6 CI 11725 Hansa Yellow 3R Food Orange 3 1 Using in hair dye 7 CI 11920 products is forbidden. Solvent Red 3 3 Using in hair dye 8 CI 12010 products is forbidden. Pigment Red 4 1 1. Limited content: D&C Red No. 36 3% 9 CI 12085 Permanent Red 2. Using in hair dye products is forbidden. 1 Colour Index Number/ Scope of Number Alias Name Restriction Ingredient Name Application Pigment Red 3 4 10 CI 12120 D&C Red No. -

Technological Applications of Natural Colorants in Food Systems: a Review
foods Review Technological Applications of Natural Colorants in Food Systems: A Review Ivan Luzardo-Ocampo 1 , Aurea K. Ramírez-Jiménez 2 , Jimena Yañez 2 , Luis Mojica 3 and Diego A. Luna-Vital 2,* 1 Instituto de Neurobiología, Universidad Nacional Autónoma de México (UNAM), Santiago de Querétaro, QRO 76230, Mexico; [email protected] 2 Tecnologico de Monterrey, School of Engineering and Science, Avenida Eugenio Garza Sada 2501 Sur, Monterrey, N. L. 64849, Mexico; [email protected] (A.K.R.-J.); [email protected] (J.Y.) 3 Tecnología Alimentaria, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ), A. C., Camino Arenero #1227 Col. El Bajío, Zapopan, JAL 45019, Mexico; [email protected] * Correspondence: [email protected] Abstract: Natural colorants have emerged as an alternative to their synthetic counterparts due to an existing health concern of these later. Moreover, natural-food colorants are a renewable option providing health benefits and interesting technological and sensory attributes to the food systems containing them. Several sources of natural colorants have been explored aiming to deliver the required wide color range demanded by consumers. This review aimed to compare and discuss the technological applications of the main natural-food colorants into food system in the last six years, giving additional information about their extraction process. Although natural colorants are promising choices to replace synthetic ones, optimization of processing conditions, research on new sources, and new formulations to ensure stability are required to equate their properties to their Citation: Luzardo-Ocampo, I.; synthetic counterparts. Ramírez-Jiménez, A.K.; Yañez, J.; Mojica, L.; Luna-Vital, D.A. -
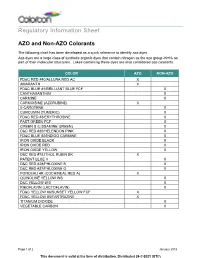
Regulatory Information Sheet AZO and Non-AZO Colorants
Regulatory Information Sheet AZO and Non-AZO Colorants The following chart has been developed as a quick reference to identify azo dyes. Azo dyes are a large class of synthetic organic dyes that contain nitrogen as the azo group -N=N- as part of their molecular structures. Lakes containing these dyes are also considered azo colorants. COLOR AZO NON-AZO FD&C RED #40/ALLURA RED AC X AMARANTH X FD&C BLUE #1/BRILLIANT BLUE FCF X CANTHAXANTHIN X CARMINE X CARMOISINE (AZORUBINE) X ß-CAROTENE X CURCUMIN (TUMERIC) X FD&C RED #3/ERYTHROSINE X FAST GREEN FCF X GREEN S (LISSAMINE GREEN) X D&C RED #30/HELENDON PINK X FD&C BLUE #2/INDIGO CARMINE X IRON OXIDE BLACK X IRON OXIDE RED X IRON OXIDE YELLOW X D&C RED #7/LITHOL RUBIN BK X PATENT BLUE V X D&C RED #28/PHLOXINE B X D&C RED #27/PHLOXINE O X PONCEAU 4R (COCHINEAL RED A) X QUINOLINE YELLOW WS X D&C YELLOW #10 X RIBOFLAVIN (LACTOFLAVIN) X FD&C YELLOW #6/SUNSET YELLOW FCF X FD&C YELLOW #5/TARTRAZINE X TITANIUM DIOXIDE X VEGETABLE CARBON X Page 1 of 2 January 2018 This document is valid at the time of distribution. Distributed 24-?-2021 (UTC) The information contained herein, to the best of our knowledge is true and accurate. Any recommendations or suggestions are made without warranty or guarantee, since the conditions of use are beyond our control. Any information contained herein is intended as a recommendation for use of our products so as not to infringe on any patent. -

Dyes, Colors & Pigements
Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org DYES , COLORS & PIGMENTS Writing By TAREK ISMAIL KAKHIA 0 Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org Natural dye Skeins of wool colored with natural plant dyes. Contents : 1 Origins 2 Processes 3 Common dyestuffs o 3.1 Reds and pinks o 3.2 Oranges o 3.3 Yellows o 3.4 Greens o 3.5 Blues o 3.6 Purples o 3.7 Browns o 3.8 Greys and blacks o 3.9 Lichen o 3.10 Fungi 4 Luxury dyestuffs o 4.1 Royal purple o 4.2 Crimson and scarlet o 4.3 The rise of formal black 5 Decline and rediscovery 6 Notes 7 References 1 Copyright © Tarek Kakhia. All rights reserved. http://tarek.kakhia.org - Introduction : Natural dyes are dyes or colorants derived from plants, invertebrates, or minerals. The majority of natural dyes are vegetable dyes from plant sources – roots, berries, bark, leaves, and wood — and other organic sources such as fungi and lichens. Archaeologists have found evidence of textile dyeing dating back to the Neolithic period. In China, dyeing with plants, barks and insects has been traced back more than 5,000 years. The essential process of dyeing changed little over time. Typically, the dye material is put in a pot of water and then the textiles to be dyed are added to the pot, which is heated and stirred until the color is transferred. Textile fiber may be dyed before spinning (dyed in the wool), but most textiles are yarn- dyed or piece-dyed after weaving. -

Food Colours and Their Chemistry
Journal of Agricultural Engineering and Food Technology p-ISSN: 2350-0085; e-ISSN: 2350-0263; Volume 6, Issue 2; April-June, 2019, pp. 129-132 © Krishi Sanskriti Publications http://www.krishisanskriti.org/Publication.html Food Colours and their Chemistry Dr Namita Gandhi Assistant Professor, Department Of Chemistry, Deshbandhu College (University of Delhi) Delhi E-mail: [email protected] Abstract—There is a saying that ‘We eat with our eyes first’. White bread was prepared by mixing flour with Colours play an important role in our life as well as food. Food alum(whitening agent) colours influence our perception of its taste. This paper details about history of food colouring, its need and its chemistry. Natural food Wine was artificially coloured. colours like chlorophyll, turmeric, saffron, carotenoids etc. have been used for centuries. With time, synthetic food colours like allura red, Silver, gold or copper were even used to garnish the food tartrazine, brilliant blue, quinoline yellow have been derived from in bright colors. chemicals. Now-a-days due to increasing competition, packaged and processed food companies are using food colours to make their Aztec people living in valley of Mexico, used to cultivate product visibly attractive and saleable. It is safe to add permitted an insect, known as cochineal from which dye was colours in prescribed limits to the food .Though we have regulatory extracted. These days it is named as Natural Red 4 and bodies like Food Safety and Standards Authority of India (FSSAI) to used as food colour. check and balance the food additives but there are concerns regarding the use of artificial colours. -

Validation of an Analytical Method for Determination of Eight Food Dyes in Beverage and Fruit Roll-Ups by Ion-Pair HPLC-DAD
Journal of Shahrekord University of Medical Sciences Archivedoi:10.34172/jsums.2020.20 of SID 2020;22(3):126-134 http://j.skums.ac.ir Original Article Validation of an analytical method for determination of eight food dyes in beverage and fruit roll-ups by ion-pair HPLC-DAD Maryam Zahedi1 ID , Amir Shakerian2* ID , Ebrahim Rahimi1 ID , Reza Sharafati Chaleshtori2,3 ID 1Department of Food Hygiene, Faculty of Veterinary Medicine, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran. 2Research Center of Nutrition and Organic Products (R.C.N.O.P), Shahrekord Branch, Islamic Azad University, Shahrekord, Iran. 3Research Center for Biochemistry and Nutrition in Metabolic Disease, Kashan University of Medical Sciences, Kashan, Iran. *Corresponding Author: Amir Shakerian, Research Center of Nutrition and Organic Products (R.C.N.O.P), Shahrekord Branch, Islamic Azad University, Shahrekord, Iran, Tel: +98-38-2244515, Email: [email protected], [email protected] Abstract Background and aims: Synthetic dyes are widely used as food additives to avoid the loss of original dye in processed foods and to make the foods more attractive to consumers. For simultaneous determination of 8 most commonly used synthetic colors in beverage and foodstuff, an efficient, selective, and sensitive method is suggested. Methods: To analyze food colors in different beverages and fruit roll-ups, a method using Ion-pair high-performance liquid chromatography with diode array detector was suggested and validated. The separation of dyes from beverage and foodstuff was done by solid-phase extraction (SPE). The ultrasound-assisted solvent extraction method was used to extract dye from fruit roll-ups. -

Wo 2009/089496 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date PCT (10) International Publication Number 16 July 2009 (16.07.2009) WO 2009/089496 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every CIlD 3/40 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT,AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, (21) International Application Number: CH, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, PCT/US2009/030667 EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, (22) International Filing Date: 9 January 2009 (09.01.2009) LR, LS, LT, LU, LY,MA, MD, ME, MG, MK, MN, MW, (25) Filing Language: English MX, MY,MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY,TJ, (26) Publication Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW (30) Priority Data: 61/020,061 9 January 2008 (09.01.2008) US (84) Designated States (unless otherwise indicated, for every 61/044,442 11 April 2008 (11.04.2008) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (71) Applicant (for all designated States except US): RE¬ ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), VEAL SCIENCES, LLC [US/US]; 11412 Bee Caves European (AT,BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, Rd., Ste.300, Austin, TX 78738 (US). -

Food Colorants: Their Past, Present, and Future
This is a repository copy of Food colorants: their past, present, and future. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/126336/ Version: Accepted Version Article: Coultate, T and Blackburn, RS orcid.org/0000-0001-6259-3807 (2018) Food colorants: their past, present, and future. Coloration Technology, 134 (3). pp. 165-186. ISSN 1472-3581 https://doi.org/10.1111/cote.12334 (c) 2018 The Authors. Coloration Technology (c) 2018 Society of Dyers and Colourists. This is the peer reviewed version of the following article: Food colorants: their past, present, and future Coultate, T and Blackburn, RS, Coloration Technology. which has been published in final form at https://doi.org/10.1111/cote.12334. This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Self-Archiving. Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Food colorants: their past, present, and future Tom Coultate* Leighton Buzzard, Bedfordshire, LU7 2RW; *e-mail: [email protected] Richard S. -

E100 Curcumin (From Turmeric) E101 Riboflavin (Vitamin B2), Formerly Called Lactoflavin E101a Riboflavin-5'-Phosphate E102 Tartr
E100 Curcumin (from turmeric) E235 Natamycin, Pimaracin E366 Potassium fumarate E101 Riboflavin (Vitamin B2), formerly called E236 Formic acid E367 Calcium fumarate lactoflavin E237 Sodium formate E368 Ammonium fumarate E101a Riboflavin-5'-Phosphate E238 Calcium formate E370 1,4-Heptonolactone E102 Tartrazine (FD&C Yellow 5) E239 Hexamine (hexamethylene tetramine) E380 Triammonium citrate E103 Alkannin[11] E240 Formaldehyde E381 Ammonium ferric citrate E104 Quinoline Yellow WS E242 Dimethyl dicarbonate E383 Calcium glycerylphosphate E105 Fast Yellow AB E249 Potassium nitrite E384 Isopropyl citrate E106 Riboflavin-5-Sodium Phosphate E250 Sodium nitrite E385 Calcium disodium ethylene diamine E107 Yellow 2G E251 Sodium nitrate (Chile saltpetre) tetraacetate, (Calcium disodium EDTA) E110 Sunset Yellow FCF (Orange Yellow S, FD&C E252 Potassium nitrate (Saltpetre) E386 Disodium ethylene diamine tetraacetate Yellow 6) E260 Acetic acid (preservative) (Disodium EDTA) E111 Orange GGN E261 Potassium acetate (preservative) E387 Oxystearin E120 Cochineal, Carminic acid, Carmine (Natural E262 Sodium acetates (i) Sodium acetate (ii) Sodium E388 Thiodipropionic acid Red 4) diacetate (sodium hydrogen acetate) E389 Dilauryl thiodipropionate E121 Citrus Red 2 E263 Calcium acetate (preservative) E390 Distearyl thiodipropionate E122 Carmoisine (azorubine) E264 Ammonium acetate E391 Phytic acid E123 Amaranth (FD&C Red 2) E265 Dehydroacetic acid E392 Extracts of rosemary E124 Ponceau 4R (Cochineal Red A, Brilliant Scarlet E266 Sodium dehydroacetate E399 Calcium