A Method to Recognize Universal Patterns in Genome Structure Using Hi-C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The 26Th Society for Animation Studies Annual Conference Toronto
Sheridan College SOURCE: Sheridan Scholarly Output, Research, and Creative Excellence The Animator Conferences & Events 6-16-2014 The Animator: The 26th oS ciety for Animation Studies Annual Conference Toronto June 16 to 19, 2014 Society for Animation Studies Paul Ward Society for Animation Studies Tony Tarantini Sheridan College, [email protected] Follow this and additional works at: http://source.sheridancollege.ca/conferences_anim Part of the Film and Media Studies Commons SOURCE Citation Society for Animation Studies; Ward, Paul; and Tarantini, Tony, "The Animator: The 26th ocS iety for Animation Studies Annual Conference Toronto June 16 to 19, 2014" (2014). The Animator. 1. http://source.sheridancollege.ca/conferences_anim/1 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 License. This Book is brought to you for free and open access by the Conferences & Events at SOURCE: Sheridan Scholarly Output, Research, and Creative Excellence. It has been accepted for inclusion in The Animator by an authorized administrator of SOURCE: Sheridan Scholarly Output, Research, and Creative Excellence. For more information, please contact [email protected]. THANK YOU TO OUR SPONSORS THE ANIMATOR THEThe 26th Society forANIMATOR Animation Studies Annual Conference TheToronto 26 Juneth Society 16 to 19, 2014 for www.theAnimation animator2014.com Studies @AnimatorSAS2014 Annual Conference Toronto June 16 to 19, 2014 • www.the animator2014.com • @AnimatorSAS2014 WELCOME Message from the President Animation is both an art and skill; it is a talent that is envied the world over. Having a hand in educating and nurturing some of the finest animators in the world is something for which Sheridan is exceptionally proud. -

Segmentation-Free Online Arabic Handwriting Recognition
International Journal of Pattern Recognition and Arti¯cial Intelligence Vol. 25, No. 7 (2011) 1009À1033 #.c World Scienti¯c Publishing Company DOI: 10.1142/S0218001411008956 SEGMENTATION-FREE ONLINE ARABIC HANDWRITING RECOGNITION FADI BIADSY Computer Science, Columbia University New York, NY 10027, USA [email protected] RAID SAABNI Computer Science, Ben-Gurion University of The Negev Beer-Sheva, 84105, Israel Triangle Research & Development Center Kafr Qara, 30075, Israel [email protected] JIHAD EL-SANA Computer Science, Ben-Gurion University of The Negev Beer-Sheva, 84105, Israel [email protected] Arabic script is naturally cursive and unconstrained and, as a result, an automatic recognition of its handwriting is a challenging problem. The analysis of Arabic script is further complicated in comparison to Latin script due to obligatory dots/stokes that are placed above or below most letters. In this paper, we introduce a new approach that performs online Arabic word recog- nition on a continuous word-part level, while performing training on the letter level. In addition, we appropriately handle delayed strokes by ¯rst detecting them and then integrating them into the word-part body. Our current implementation is based on Hidden Markov Models (HMM) and correctly handles most of the Arabic script recognition di±culties. We have tested our implementation using various dictionaries and multiple writers and have achieved encouraging results for both writer-dependent and writer-independent recognition. Keywords: Online handwriting recognition; Arabic; HMM. 1. Introduction Keyboards and electronic mice may not endure as the prevalent means of human- computer interfacing. Devices such as digital tablets, hand-held computers, and mobile technology, provide signi¯cant opportunities for alternative interfaces that work in forms smaller than the traditional keyboard and mouse. -

Song List by Member
song artist album label dj year-month-order leaf house animal collective sung tongs 2004-08-02 bebete vaohora jorge ben the definitive collection 2004-08-08 amor brasileiro vinicius cantuaria tucuma 2004-08-09 crayon manitoba up in flames 2004-08-10 transit fennesz venice 2004-08-11 cold irons bound bob dylan time out of mind 2004-08-13 mini, mini, mini jacques dutronc en vogue 2004-08-14 unspoken four tet rounds 2004-08-15 dead homiez ice cube kill at will 2004-08-16 forever's no time at all pete townsend who came first 2004-08-17 mockingbird trailer bride hope is a thing with feathers 2004-08-18 call 1-800 fear lali puna faking the books 2004-08-19 vuelvo al sur gotan project la revancha del tango 2004-08-21 brick house commodores pure funk polygram tv adam 1998-10-09 louis armstrong - the jazz collector mack the knife louis armstrong edition laserlight adam 1998-10-18 harry and maggie swervedriver adam h. 2012-04-02 dust devil school of seven bells escape from desire adam h. 2012-04-13 come on my skeleton plug back on time adam h. 2012-09-05 elephant tame impala elephant adam h. 2012-09-09 day one toro y moi everything in return adam h. 2014-03-01 thank dub bill callahan have fun with god adam h. 2014-03-10 the other side of summer elvis costello spike warner bros. adam s (#2) 2006-01-04 wrong band tori amos under the pink atlantic adam s (#2) 2006-01-12 Baby Lemonade Syd Barrett Barrett Adam S. -

Norman Mclaren: Between the Frames
Dobson, Nichola. "Influential Arts." Norman McLaren: Between the Frames. London: Bloomsbury Academic, 2017. 109–132. Animation: Key Films/ Filmmakers. Bloomsbury Collections. Web. 30 Sep. 2021. <http://dx.doi.org/10.5040/9781501328800.ch-004>. Downloaded from Bloomsbury Collections, www.bloomsburycollections.com, 30 September 2021, 11:41 UTC. Copyright © Nichola Dobson 2018. You may share this work for non-commercial purposes only, provided you give attribution to the copyright holder and the publisher, and provide a link to the Creative Commons licence. 109 Chapter 4 I NFLUENTIAL ARTS McLaren found inspiration and infl uence in many aspects of his life, with early talent undoubtedly informed, by his family and their interior decorating busi- ness. A fl air for this could be seen in early examples of drawing and design completed while he attended Stirling High School and then Glasgow School of Art. It has been outlined elsewhere 1 that he was infl uenced by early interests in music and dance and these rather substantive aspects of interest are examined in more depth later in this chapter. Th is section considers some of the earlier, formative experiences that remained with him throughout his career, from his Art School education, through to some of the pioneering animators, and which he would later cite as being so infl uential to him. Th e chapter begins with a discussion of his painting habit; something which would remain with him throughout his life and would even be used as a meta- phor when describing his process of direct animation: ‘And so my militant phi- losophy is this: to make with a brush on canvas is a simple and direct delight – to make a movie should be the same’. -

Pdfs That Request Users Contact the Drawing Information Systems Group
MIT’s The Weather Oldest and Largest Today: Cloudy, 40°F (4°C) Tonight: Cloudy, late showers, Newspaper 50°F (10°C) Tomorrow: Rain, High 57°F (14°C) http://tech.mit.edu/ Details, Page 2 Volume 128, Number 61 Cambridge, Massachusetts 02139 Tuesday, December 9, 2008 Initial Dining Proposal Includes Possible Automatic Meal Charge By Austin Chu mitted its first set of proposals to be habits. During the term, this money STAFF REPORTER evaluated by an outside consultant. could be applied toward meals at Amid reports that the Blue Rib- One of these proposals includes a house dining locations and on cam- bon Committee on Dining is consid- “minimum nutritional fee” to en- pus restaurants, gift cards for Star ering the possibility of an automatic courage students to invest in their Market or Trader Joe’s, or fraternity, meal charge for most undergradu- nutritional well being. sorority, or independent living group ates, the Undergraduate Association This proposal, variously de- dining membership fees. Senate last night passed a bill de- scribed over the past week and con- The revelation that the Blue Rib- manding more transparency in the firmed by members of the commit- bon Committee on Dining had al- committee’s deliberations. tee, would require undergraduates ready sent proposals to an outside The Blue Ribbon Committee on to pay a certain minimum amount consultant prompted students to call Dining, formed in the fall of 2007 by for food-related expenses per term. for more transparency. Currently, former Dean for Student Life Larry By making this amount a sunk cost, members of the committee are not G. -

Pittiplatsch 3000 #11, Juni 2005
#11 pop.kultur.zine juni05 • 2€euro Spezial Du und viele von deinen gesichtslosen Internet-Freunden Interviews Frank Black • Kimya Dawson • Schwervon! • Archie Müller • Körperhaarschmuck Stories Stereolab • Libertines • Morrissey • Weezer • John Michael McCarthy • Twin Peaks • Die Kunst des Dissens • (Anti-)Pop und Kunst so put that record on and dance until the sadness is gone Wer dieses Heft in den Händen hält und auch Leidenschaft ist. Es hat sicher auch etwas damit zu tun, schon früher mal die eine oder andere Ausgabe von dass Säm und ich uns kaum mehr sehen. Auf keinen Pitti Platsch 3000 gelesen hat, kann es wahrscheinlich Fall hat es etwas mit Erwachsenwerden oder so einem kaum glauben, noch einmal (in Printform) von uns zu Schwachsinn zu tun. Indes, wie soll man so ein auf hören. Noch kann ich es glauben. Was ist seitdem Enthusiasmus bauendes Projekt vorantreiben? passiert? Nun, sehr viel und doch nichts. Genauer Jedenfalls haben wir zu danken: den Autoren dieser darauf einzugehen würde ein eigenes Heft, wenn nicht Ausgabe, ohne die diese nicht möglich gewesen wäre. Buch, beanspruchen, schlechte Laune verbreiten und Elvira für die Layout-Arbeit. Unseren Abonnenten, den am Ende doch niemanden interessieren. Deshalb von Leuten, die NICHT angeblich im Moment wirklich kein mir dieses Mal kein Seelen-FKK, keine Selbstdarstellung, Geld hätten und sich anschließend drei Bier vom Tresen kein Fanzine-typischer Ego-Kram – nur das, wovon und geholt haben, und natürlich denen, die immer wieder wofür wir hier leben, womit wir unsere Beziehungen gefragt haben, wann es denn ein neues Heft gibt. Vor strapazieren, woran wir unser Geld, unsere Zeit und allem ihnen. -

The Film-Work of Norman Mclaren in the Context of His Objectives
The Film-Work Norman McLaren Terence Dobson A thesis submitted in accordance with the requirements for the Degree of Doctor of Philosophy in the University of Canterbury University of Canterbury 1994 Contents page Abstract iii List of Illustrations iv Preface v Introduction vii Part One Chapter One An Informative Drawing 1 Chapter Two The Early Years 23 Chapter Three McLaren at the G PO Film Unit 57 Part Two Chapter Four New York Interlude 103 Chapter Five Canada 128 Part Three Chapter Six Technical Processes 170 Chapter Seven Confluence and Conflict in Synchromy 197 Chapter Eight Venus and Mars 218 Conclusion 237 Chronology 248 Bibliography 249 Interviews, Films and Recordings 281 ill Abstract This thesis examines the film-work of Norman Mclaren in the context of his objectives. The thesis is divided into three parts, based on chronological divisions in McLaren's life. The first part deals with McLaren's formative years in Scotland and England and examines his early exposure to the social, artistic and institutional influences that were to shape his filmic output. The second part deals with Mclaren's maturation in the USA and Canada. His reaction to the contrasting working environment he found in each of the two countries is examined. In order to show McLaren's development more clearly, both parts one and two are based on a chronological sequencing. The third part of the thesis examines specific issues in relation to Mclaren and his work and as such is concerned principally with his mature output. McLaren's films contain incongruities, conflicts and apparent inconsistencies. -

Beta Theta Pi Tosee National Charter
VOL. 104 No.42 reeA STUDENTPUBLICATIONOFWASHINGTONSTATEUNIVERSITY SPORTS FriQay, October 17, 1997 No. 13 WSU hosts Cal in U.S. could stop worldwide Weather 21 Sports 12 Homecoming game religious persecution Borderline 15 Classifieds 26 Page 5 www.online-green.com Page 11 A Beta Theta Pi tosee national charter By ANNIE MORROW their new situation. also said the fraternity's road to The Daily Evergreen "(Richard) Phennager comes in homelessness began in January, here and says, 'Get the hell out!'" when the national fraternity organi- Former Beta Theta Pi members former Beta Theta Pi member Ken zation issued a set of guidelines sat on their front steps Thursday dis- Colman said of the fraternity district called the Reconstruction Plan. cussing where to get apartments, chief's recent decision to dismember The four-year plan was a way for how they would pay for them and the group and close their house the fraternity to redeem itselfafter the how, in 21 hours. they would vacate down. alcohol violations. a fraternity house that had been in Colman was frustrated and voiced A senior member remembered use for 58 years. the feelings of his brothers. what the house was like whenhe was The chapter received a letter "Something that you love dearly a freshman. Wednesday night, hand-delivered by got pulled out from under you," he "It was the 'Animal House' in the alumni and accompanied by security said. movie," he said under condition of guards. The contents of the letter The charter of the chapter was anonymity. explained their charter had been suspended in January 1996, because Broken beer bottles scattered revoked and the members would be of conduct issues related to alcohol throughout the house were, at one STAFF PHOTO BY NICOLE WALLA forced to move out of their house, problems, Phennager explained. -

Wallop 10.99
Recording Artist Recording Title Price !!! (chk Chk Chk) Wallop 10.99 (sandy) Alex G House Of Sugar 10.99 10CC I'm Not In Love - Essential Collection 5.99 1975 Notes On A Conditional Form 11.99 1975 A Brief Inquiry Into Online Relationships 7.99 2pac The Best Of Part 1 - Thug 5.99 2pac The Best Of Part 2 - Life 6.99 50 Cent Best Of 7.99 65daysofstatic Replicr 2019 11.99 6lack East Atlanta Love Letter 6.99 A Tribe Called Quest The Low End Theory 5.99 A Tribe Called Quest We Got It From Here Thank You 4 Your Service 11.99 A Tribe Called Quest People's Instinctive Travels... 25th Anniversary 7.99 A Tribe Called Quest Midnight Marauders 5.99 Abba Gold - Greatest Hits- Always Oversticker 7.99 ABC The Lexicon Of Love 6.99 AC/DC Highway To Hell 9.99 AC/DC Stiff Upper Lip 7.99 AC/DC High Voltage 9.99 AC/DC If You Want Blood You've Got It 9.99 AC/DC For Those About To Rock ( We Salute You) 9.99 AC/DC Back In Black 9.99 Adele 25 10.99 Adele 21 10.99 Adele 19 7.99 Aerosmith Young Lust - The Anthology 7.99 Africa Express Presents Egoli 9.99 Agnes Obel Philharmonics 7.99 Agnes Obel Late Night Tales 10.99 Agnes Obel Myopia 11.99 Agnes Obel Aventine 9.99 Agnes Obel Citizen Of Glass 7.99 Ags Connolly Wrong Again 10.99 Ags Connolly Nothin' Unexpected 10.99 Airto Moreira & Flora Purim Brazil Usa - Brazilian Music In The Usa In The 1970s12.99 Al Green I'm Still In Love With You 8.99 Alabaster Deplume To Cy & Lee - Instrumentals - Vol 1 11.99 Alanis Morissette The Collection 5.99 Albert King The Very Best Of 6.99 Albert King Born Under A Bad Sign 6.99 Aldous -
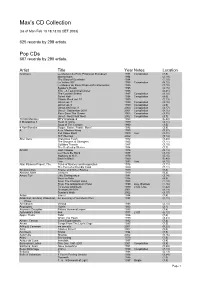
Max's CD Collection
Max's CD Collection (as of Mon Feb 10 18:13:08 CET 2003) 625 records by 298 artists. Pop CDs 607 records by 290 artists. Artist Title Year Notes Location (Various) Le Meilleur du Rock Progressif Européen 1994 Compilation (7,5) Mannerisms 1994 (2,19) The Glory of Gershwin 1994 (1,10) La Yellow 357 1995 Compilation (8,12) Le Meileur du Rock Progressif Instrumental 1995 (7,10) Supper's Ready 1995 (2,15) XTC - A Testimonial Dinner 1995 (2,21) The Cocktail Shaker 1997 Compilation (8,14) Select Hot! 1998 Compilation (4,6) Classic Rock vol. 10 1999 (2,13) Uncut vol. 7 1999 Compilation (2,16) Uncut vol. 9 1999 Compilation (4,9) Uncut 2000 vol. 3 2000 Compilation (4,17) Uncut - September 2001 2001 Compilation (8,15) Rock Save The Queen 2002 Compilation (9,25) Uncut - Neat Neat Neat 2002 Compilation (9,7) 10,000 Maniacs MTV Unplugged 1993 (6,42) 3 Mustaphas 3 Heart of Uncle 1989 (2,11) Soup of The Century 1990 (3,5) 4 Non Blondes Bigger, Better, Faster, More! 1992 (3,9) A A vs. Monkey Kong 1999 (9,27) Exit Stage Right 2000 Live (9,21) Hi-Fi Serious 2002 (9,14) Abel Ganz Gratuitous Flash 1982 (3,20) The Dangers of Strangers 1985 (7,11) Gullibles Travels 1987 (7,10) The Deafening Silence 1994 (7,7) AC/DC High Voltage 1976 (5,14) Let There Be Rock 1977 (3,13) Highway to Hell 1979 (5,15) Back in Black 1980 (6,40) Live 1992 Live (4,15) Alan Parsons Project, The Tales of Mystery and Imagination 1976 (7,11) The Turn of a Friendly Card 1980 (7,11) All About Eve Scarlet and Other Stories 1989 (7,3) Almond, Marc Jacques 1989 (9,4) Amos, Tori Little Earthquakes 1991 (3,14) Boys for Pele 1996 (4,9) From The Choirgirl Hotel 1998 (1,13) From The Glastonbury Hotel 1999 Live, Bootleg (1,16) To Venus And Back 1999 2 CD. -

The Lost Generation How UK Post-Rock Fell in Love with the Moon, and a Bunch of Bands Nobody Listened to Defined the 1990S by Nitsuh Abebe
Pitchfork | http://pitchforkmedia.com/features/weekly/05-07-11-lost-generation.shtml 11 July 2005 The Lost Generation How UK post-rock fell in love with the moon, and a bunch of bands nobody listened to defined the 1990s by Nitsuh Abebe his decade’s indie-kid rhetoric is all about excitement, all about fun, all about fierce. TThe season’s buzz tour pairs ... with Soundsystem, scrappy globo-pop with the kind of rock disco that tries awfully hard to blow fuses. The venues they don’t hit will play host to a new wave of stylish guitar bands, playing stylish uptempo pop, decamping to stylish afterparties. Bloggers will chatter about glittery chart hits, rock kids will buy vintage metal t-shirts and act like heshers, eggheads will rave about the latest in spazzed-out noise, and everyone will keep talking about dancing, right down to the punks. Yeah, there are more exceptions than there are examples—when aren’t there, dude?—but the vibe is all there: We keep talking like we want action, like we want something explosive. The overriding vibe of the s—serene, cerebral, dreamy—was anything but explosive. Blame grunge for that one. Just a few years into the decade, and all that muddy rock attitude and fuzzy alterna-sound had bubbled up into the mainstream, flooding the scene with new kids, young kids, even (gasp!) fratboys. Big kids in flannel, freshly enamored of rock’s “aggres- sion” and “rebellion”—they were rock jocks, they tried to start mosh pits at Liz Phair shows. So where was a refined little smarty-pants indie snob to turn? Well: Why not something spacey and elegant? Why not sit comfortably home, stoned or non-stoned, dropping out into a dreamier little world of sound? It had been a few years since My Bloody Valentine released Loveless, and there were plenty of similarly floaty shoegazer bands to catch up on. -

PDF (925.27 Kib)
,- .'t' ] '; RE M's Up Shows: Avante-garde, ambient art music continued from page 7 some of their best shows at Tip's, and this that really sounds like come to John Zeiss night was not an exception to the rule. In fact anything REM has ever done being love they played for over five-and-a-half hours, contributing writer before. I guess the true fan songs. Stipe finally leaving the stage for good at about that I am - I have four of has always 6:30 a.m. It became evident very quickly to Great bands change over their albums on original vinyl asserted that everyone there that night why the show had time; or at least エ ィ 。 エ G セ my and the rare pre-Murmur ep he never sold out almost a month ago. The show itself philosophy. I'm probably the Chronic Town - I still yearn writes such wasn't the only only one left who still likes for the good ole sound. But songs. But amazing element to Pearl Jam from my circle of the song is undeniably catchy, with lyrics take into friends who were fans back with its "Hey! Hey!" loop, like, "I save consideration. Another when Jeremy first spoke in bouncing keyboards, classic y o u r is the fact that Galactic class. With their new release Peter Buck guitar riff and messages was just back from a The one word good work behind the set by just to hear Up, REM has changed like post Phish-show gig in which best never before.