29 Biomolecules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
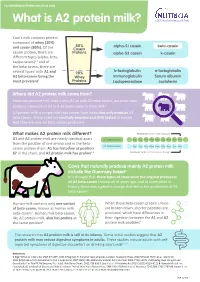
What Is A2 Protein Milk?
For Healthcare Professional use only What is A2 protein milk? Cow’s milk contains protein composed of whey (20%) 80% alpha-S1 casein beta-casein and casein (80%). Of the Casein casein protein, there are Proteins alpha-S2 casein k-casein different types (alpha, beta, kappa casein)1,2 and of the beta-casein, there are b-lactoglobulin a-lactoglobulin several types’ with A1 and 20% A2 beta-casein being the Whey Immunoglobulin Serum albumin most prevalent3 Proteins Lactoperoxidase Lactoferrin Where did A2 protein milk come from? Cows can produce milk that is only A1 or only A2 beta-casein, but most cows produce a mixture of A1 and A2 beta-casein in their milk4 A2 protein milk is simply milk that comes from cows that only produce A2 beta-casein. These cows are carefully selected and DNA tested to ensure that they are only A2 beta-casein producers What makes A2 protein milk different? Position 67(proline hinders cleavage) A1 and A2 protein milk are nearly identical apart A2 beta-casein Val Tyr Pro Phe Pro Gly Pro Iie Pro from the position of one amino acid in the beta- A1 beta-casein casein protein chain: A1 has histadine at position Val Tyr Pro Phe Pro Gly Pro Iie His 67 in the chain, and A2 protein milk has proline4,5 Position 67(histidine readily allows cleavage) Cows that naturally produce mainly A2 protein milk include the Guernsey breed4 It is thought that these types of cows were the original producers of A2 beta-casein thousands of years ago, and at some point in history there was a genetic change that led to the production of A1 beta-casein6 Human milk contains only one variant When these beta-casein protein chains of beta-casein, known as human milk are broken down, shorter peptides are beta-casein7. -

Dispensing of Vitamin Products by Retail Pharmacies in South Africa: Implications for Dietitians
South African Journal of Clinical Nutrition 2016; 29(4):133–138 http://dx.doi.org/10.1080/16070658.2016.1219468 SAJCN ISSN 1607-0658 EISSN 2221-1268 Open Access article distributed under the terms of the © 2016 The Author(s) Creative Commons License [CC BY-NC 3.0] http://creativecommons.org/licenses/by-nc/3.0 RESEARCH Dispensing of vitamin products by retail pharmacies in South Africa: Implications for dietitians Ilse Trutera* and Liana Steenkampb a Department of Pharmacy, Drug Utilisation Research Unit (DURU), Nelson Mandela Metropolitan University, Port Elizabeth, South Africa b HIV & AIDS Research Unit, Nelson Mandela Metropolitan University, Port Elizabeth, South Africa *Corresponding author, email: [email protected] Objective: The objective of this study was to analyse the dispensing patterns of vitamins (Anatomical Therapeutic Chemical (ATC) group A11) over a one-year period in a group of community pharmacies in South Africa. Design and setting: A retrospective drug utilisation study was conducted on community pharmacy electronic dispensing records in South Africa recorded in 2013. Outcome measures: All products for ATC subgroup A11 were extracted and analysed. Results: A total of 164 233 vitamin products were dispensed to 84 805 patients (62.64% female patients). Males received on average 2.09 (SD = 2.63) vitamin products per year, compared to 1.84 (SD = 2.13) products for females. Ergocalciferol (A11CC01) was the most often dispensed (37.48% of all vitamin products), followed by plain Vitamin B-complex products (A11EA00) accounting for 32.77%. Ergocalciferol (vitamin D2) is only available on prescription (50 000 IU tablets or 50 000 IU/ml oily drops) in South Africa. -

Casein Proteins As a Vehicle to Deliver Vitamin D3
Casein Proteins as a Vehicle to Deliver Vitamin D3: Fortification of Dairy Products with vitamin D3 and Bioavailability of Vitamin D3 from Fortified Mozzarella Cheese Baked with Pizza by Banaz Al-khalidi A thesis submitted in conformity with the requirements for the degree of Master of Science Graduate Department of Nutritional Sciences University of Toronto © Copyright by Banaz Al-khalidi (2012) Casein proteins as a vehicle to deliver vitamin D3: Fortification of dairy products with vitamin D3 and Bioavailability of vitamin D3 from fortified Mozzarella cheese baked with pizza Banaz Al-khalidi Master of Science Department of Nutritional Sciences, Faculty of Medicine University of Toronto 2012 ABSTRACT Current vitamin D intakes in Canada are inadequate. The extension of vitamin D fortification to additional foods may be an effective and appropriate strategy for increasing vitamin D intakes in the general population. Cheese is potentially an ideal candidate for vitamin D fortification. We introduce the potential use of casein proteins as a vehicle for vitamin D3 fortification in industrially made cheeses where we found that over 90% of vitamin D3 added to milk was retained in both Cheddar and Mozzarella cheeses. Use of casein proteins for vitamin D3 fortification did not fully prevent vitamin D3 loss into whey. However the loss was minimized to approximately 8%. We then show that vitamin D3 is bioavailable from fortified Mozzarella cheese baked with pizza suggesting that the high temperature baking process does not significantly breakdown vitamin D3. Our findings could have important implications in increasing fortified food options for Canadians. ii TABLE OF CONTENTS ABSTRACT .................................................................................................................................. -

Folic Acid, Pyridoxine, and Cyanocobalamin Combination
ORIGINAL INVESTIGATION Folic Acid, Pyridoxine, and Cyanocobalamin Combination Treatment and Age-Related Macular Degeneration in Women The Women’s Antioxidant and Folic Acid Cardiovascular Study William G. Christen, ScD; Robert J. Glynn, ScD; Emily Y. Chew, MD; Christine M. Albert, MD; JoAnn E. Manson, MD Background: Observational epidemiologic studies indi- and visually significant AMD, defined as confirmed in- cate a direct association between homocysteine concentra- cident AMD with visual acuity of 20/30 or worse attrib- tion in the blood and the risk of age-related macular degen- utable to this condition. eration (AMD), but randomized trial data to examine the effect of therapy to lower homocysteine levels in AMD are Results:Afteranaverageof7.3yearsoftreatmentandfollow- lacking. Our objective was to examine the incidence of AMD up, there were 55 cases of AMD in the combination treat- in a trial of combined folic acid, pyridoxine hydrochloride ment group and 82 in the placebo group (relative risk, 0.66; (vitamin B6), and cyanocobalamin (vitamin B12) therapy. 95% confidence interval, 0.47-0.93 [P=.02]). For visually significant AMD, there were 26 cases in the combination Methods: We conducted a randomized, double-blind, treatment group and 44 in the placebo group (relative risk, placebo-controlled trial including 5442 female health care 0.59; 95% confidence interval, 0.36-0.95 [P=.03]). professionals 40 years or older with preexisting cardio- vascular disease or 3 or more cardiovascular disease risk Conclusions: These randomized trial data from a large factors. A total of 5205 of these women did not have a cohort of women at high risk of cardiovascular disease diagnosis of AMD at baseline and were included in this indicate that daily supplementation with folic acid, pyri- analysis. -

Applications
TN-1175 APPLICATIONS Xianrong (Jenny) Wei A Simple and Effective Method for HPLC Quantification Jenny is a Senior Scientist in Phenomenex’s PhenoLogix of Simultaneous Vitamin B1 (Thiamine Diphosphate) and applications laboratory. Vitamin B6 (Pyridoxal 5-Phosphate) from Whole Blood Xianrong (Jenny) Wei, Sean Orlowicz, Erica Pike, and Brian Rivera Phenomenex, Inc., 411 Madrid Ave., Torrance, CA 90501 USA Abstract The purpose of this experiment was to develop a simple and ro- 2. 25 % NaOH was prepared by adding 10 mL of 50 % NaOH to bust method for the simultaneous analysis of biologically active 10 mL DI Water. Vitamin B1 (TDP) and Vitamin B6 (PLP) from whole blood. 3. 25 mM Na2HPO4 (pH 7) was prepared by adding 6.7 g of Na2HPO4.7H2O to 1 L of DI water. pH was adjusted with 85 % Introduction H3PO4. Water-soluble B Vitamins are important cofactors in cell metabo- 4. 250/250 mg/mL semicarbazide/glycine was prepared by lism. Two water-soluble vitamins with clinical relevance are Vita- adding 500 mg of semicarbazide and 500 mg of glycine to mins B1 and B6. Thiamine Diphosphate (TDP) is the biologically 2 mL of DI water. active form of Vitamin B1 and is required for various metabolic functions. Prolonged deficiency can cause beriberi, a debilitating Sample Preparation neurological disease1. Pyridoxal 5-phosphate (PLP) is the biolog- All extraction steps were performed while protecting the sam- ically active form of Vitamin B6 and is a coenzyme for a number ple from light and under cold conditions, such as on ice. Human of transamination reactions. -

Randomised Controlled Trial of Nutritional Supplement on Bone Turnover Markers in Indian Premenopausal Women
nutrients Article Randomised Controlled Trial of Nutritional Supplement on Bone Turnover Markers in Indian Premenopausal Women Pramod B. Umarji 1, Pankaj Verma 2, Vivek Garg 2, Marian Schini 3,* and Richard Eastell 3 1 Umarji Healthcare, Pune, Maharashtra 411045, India; [email protected] 2 Hindustan Unilever Limited R&D, Gurugram, Haryana 122002, India; [email protected] (P.V.); [email protected] (V.G.) 3 Academic Unit of Bone Metabolism, University of Sheffield, Sheffield S10 2NR, UK; r.eastell@sheffield.ac.uk * Correspondence: m.schini@sheffield.ac.uk; Tel.: +44-0114-215-9667 Abstract: Young Indian women may be at risk of poor bone health due to malnutrition. The aim of this study was to examine the effects on bone metabolism of a nutritional supplement in women aged 25 to 44. The nutritional supplement was a protein-rich beverage powder fortified with multi- micronutrients including calcium (600 mg), vitamin D (400 IU), and vitamin K (55 mcg) per daily serving, while a placebo supplement was low-protein non-fortified isocaloric beverage powder. This 6-month randomised, controlled trial showed favorable changes in bone turnover markers (decreased) and calcium homeostasis; such changes in older adults have been associated with slowing of bone loss and reduced fracture risk. For example, serum CTX decreased by about 30% and PINP by about 20% as a result of the increase in calcium intake. There were also changes in the ratio of carboxylated to undercarboxylated osteocalcin and such changes have been linked to a slowing of bone loss in older subjects. For example, the ratio increased by about 60% after 3 months as a result in the improvement in vitamin K status. -

Optimal Foods
Optimal Foods 1. Almonds: high in monounsaturated and polyunsaturated fats, with 20% of calories coming from protein and dietary fiber. Nutrients include potassium, magnesium, calcium, iron, zinc, vitamin E and an antioxidant flavonoid called amygdlin also known as laetrile. 2. Barley: Like oat bran it is high in beta-glucan fiber which helps to lower cholesterol. Nutrients include copper, magnesium, phosphorous and niacin. 3. Berries : The darker the berry the higher in anti-oxidants. Nutritionally they are an excellent source of flavonoids, especially anthocyanidins, vitamin C and both soluble and insoluble fiber. 4. Brussels Sprouts : Similar to broccoli, and a member of the cabbage family, it contains cancer fighting glucosinolates. Nutritionally it is an excellent source of vitamin C and K, the B vitamins, beta-carotene, potassium and dietary fiber. 5. Carrots: It contains the highest source of proviatamin A carotenes as well as vitamin K, biotin, vitamin C, B6, potassium, thiamine and fiber. 6. Dark Chocolate: It is rich in the flavonoids, similar to those found in berries and apples, that are more easily absorbed than in other foods. It also provides an amino acid called arginine that helps blood vessels to dilate hence regulating blood flow and helping to lower blood pressure. Choose high-quality semisweet dark chocolate with the highest cocoa content that appeals to your taste buds. 7. Dark leafy greens : Kale, arugula, spinach, mustard greens, chard, collards, etc: low calorie, anti-oxidant dense food with carotenes, vitamin C, folic acid, manganese, copper, vitamin E, copper, vitamin B6, potassium, calcium, iron and dietary fiber. Kale is a particularly excellent bioavailable source of calcium while spinach is not. -

Gluten Free Casein Free Diet
GLUTEN FREE, CASEIN FREE DIET The gluten free, casein free (GFCF) diet has been shown to be helpful for individuals with allergies to these particular foods and specifically in the management of autistic spectrum disorder (ASD). Proteins found in grain and dairy products, known as gluten and casein respectively, are believed to be poorly broken down in the digestive tracts in some people. When these proteins are not digested properly they can be absorbed intact into blood circulation. These proteins can affect the brain by crossing the blood-brain barrier and binding to opioid receptors. This can affect mood, concentration, mental performance and pain tolerance (i.e. in autistic children this will increase their pain threshold). Research has shown significant improvement in several conditions, including schizophrenia and autism, following a GFCF diet. In a survey of over 3500 parents of autistic children, it was reported that 70% found a GFCF diet improved behavior, eye contact and socialisation, concentration and learning. It is recommended to follow the GFCF diet strictly for at least 6 months to assess the benefit of this diet. Below is a list of foods containing gluten and casein that are suggested to avoid, plus a list of alternative GFCF choices. RECOMMENDED AVOID GRAINS AND • Amaranth • Baked Beans unless gluten free LEGUMES • Basmati Rice • Flours: Wheat flour, wholemeal flour, • Beans bakers flour, semolina, barley, rye • Brown Rice (avoid battered or crumbed food) • Buckwheat • Wheat including durum, semolina, • Chickpea triticale, -

A2 Milk Popularity on the Rise by BEN VERSTEEG, SEMEX SALES & PRODUCT SPECIALIST
A2 Milk Popularity on the Rise BY BEN VERSTEEG, SEMEX SALES & PRODUCT SPECIALIST A hot topic in the dairy industry today is the Beta-casein protein production is controlled by the growing popularity of A2 beta-casein milk among combination of any two of these variants (ie. A1A2) as consumers and dairy farmers. Farmers in many all cows carry two alleles. These alleles are co-dominant, regions of the world are being incentivised to meaning that cows that carry two different variants produce A2 milk to meet the growing demand in (heterozygous) will produce equal amounts of each what is considered to be a healthier alternative protein that they carry, while cows that carry two copies to conventional dairy (Zoetis, 2015). However the of the same allele (homozygous) will produce only science behind this trend remains controversial that protein (Woodford, 2007). This makes achieving and is not well understood by many consumers a homozygous A2 herd exclusively through genetic and producers. The goal of this article is to selection a possibility for dairy producers. While a present an assessment of the facts as they are quick conversion to A2 would be possible via genetic currently known and explain Semex’s A2 brand. testing and selective culling of A1 carriers, a more sound approach could be a step-wise approach of genetic Milk is composed of several solid components including selection for A2A2 sires in advance of conversion to minerals, lactose, fat and protein. There are three notable mitigate the need for A1 culling. casein milk proteins: alpha, kappa, and beta-casein - the protein of interest to us in this article (Zoetis, 2015). -

Hidden Dairy “Cheat Sheet” Business Card-Sized (Cut out and Fold in the Middle)
From kellymom.com… Hidden Dairy “Cheat Sheet” Business card-sized (cut out and fold in the middle) Personal use only. If you would like to distribute this handout to clients or patients, please visit www.kellymom.com/bookstore/handouts for more information. Dairy Ingredients and Hidden Dairy Ingredients that MAY contain milk protein: Artificial butter flavor, Butter, Butter fat, Buttermilk, Chocolate, Flavorings (natural or artificial), High protein Butter oil, Casein, Caseinates (ammonia, calcium, flour, Hot Dogs, Luncheon Meat, Margarine, Simplesse, magnesium, potassium, sodium), Cheese, Cottage Sausage, Starter Distillate. cheese, Cream, Curds, Custard, Ghee, Goat’s milk, Avoid "deli" meats, because the slicers frequently are used Half & half, Hydrolysates (casein, milk protein, protein, to cut both meat and cheese products. Also, some deli whey, whey protein), Kefir, Koumiss, Lactalbumin, meats contain dairy products. Lactalbumin phosphate, Lactoglobulin, Lactose, Lactulose, Milk (condensed, derivative, powder, dry, Kosher labeling: A product label marked Parve or evaporated, low fat, malted, non fat, protein, skim, solids, Pareve is certified dairy-free. A product with a circled whole), Milkfat, Nougat, Paneer, Pudding, Rennet “U” on the label (with NO other symbols or letters) is casein, Sour cream, Sour cream solids, Sour milk solids, Parve. A "D" or "DE" on a product label next to a circled Whey (in any form including delactosed, demineralized, "K" or circled "U" may indicate the presence of milk protein concentrate, sweet), Yogurt protein. — kellymom.com . -

Secretin and Autism: a Clue but Not a Cure
SCIENCE & MEDICINE Secretin and Autism: A Clue But Not a Cure by Clarence E. Schutt, Ph.D. he world of autism has been shaken by NBC’s broadcast connections could not be found. on Dateline of a film segment documenting the effect of Tsecretin on restoring speech and sociability to autistic chil- The answer was provided nearly one hundred years ago by dren. At first blush, it seems unlikely that an intestinal hormone Bayless and Starling, who discovered that it is not nerve signals, regulating bicarbonate levels in the stomach in response to a but rather a novel substance that stimulates secretion from the good meal might influence the language centers of the brain so cells forming the intestinal mucosa. They called this substance profoundly. However, recent discoveries in neurobiology sug- “secretin.” They suggested that there could be many such cir- gest several ways of thinking about the secretin-autism connec- culating substances, or molecules, and they named them “hor- tion that could lead to the breakthroughs we dream about. mones” based on the Greek verb meaning “to excite”. As a parent with more than a decade of experience in consider- A simple analogy might help. If the body is regarded as a commu- ing a steady stream of claims of successful treatments, and as a nity of mutual service providers—the heart and muscles are the pri- scientist who believes that autism is a neurobiological disorder, I mary engines of movement, the stomach breaks down foods for have learned to temper my hopes about specific treatments by distribution, the liver detoxifies, and so on—then the need for a sys- seeing if I could construct plausible neurobiological mechanisms tem of messages conveyed by the blood becomes clear. -

Glucagon and Gastrointestinal Motility in Relation to Thyroid-Parathyroid Function
Upsala J Med Sci 77: 183-188, 1972 Glucagon and Gastrointestinal Motility in Relation to Thyroid-Parathyroid Function HENRY JOHANSSON and ANDERS SEGERSTROM From the Department of Surgery, University Hospital, Uppsala, Sweden ABSTRACT MATERIAL Gastrointestinal propulsive motility was studied after The material consisted of 109 male albino rats (Sprague- inhgastric deposition of a non-absorbable isotope in Dawley) fed on laboratory food and with free access to rats after subcutaneous glucagon injections. Glucagon water. The animals were distributed at random in fol- administration was followed by retardation of gastric lowing series: emptying. The results indicated that the retarding effect Series I of glucagon on gastrointestinal propulsion was independent The influence of glucagon on gastrointestinal motility in of the presence of both thyroid and parathyroid tissue. intact rats. The observation period was 7 days. The hypocalcemic effect of glucagon was exerted in- dependently of the presence of thyroid tissue, Le. thyro- 1. Intact rats given 1.2 mg glucagon/kg body weight calcitonin. (GN-A, n= 6) 2. Intact rats given 4.8 mg glucagon/kg body weight (GN-B, n= 5) 3. Intact rats given 9.6 mg glucagon/kg body weight INTRODUCTION (GN-C, n= 12) 4. Intact rats given glucine buffert (GLY, n= 10). Glucagon is known to influence gastrointestinal Series I1 motility. In man, Dotevall & Kock (2) observed The influence of glucagon on gastrointestinal motility in that glucagon retarded gastrointestinal motility parathyroidectomized rats. The animals were given 4.8 independently of the hyperglucemia. In dogs, mg glucagon/kg body weight. The observation period glucagon retarded motility of the stomach ani was 90 days.