Oils of Zataria Multiflora, Artemisia Deracunculus and Mentha Piperita]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Native Or Suitable Plants City of Mccall
Native or Suitable Plants City of McCall The following list of plants is presented to assist the developer, business owner, or homeowner in selecting plants for landscaping. The list is by no means complete, but is a recommended selection of plants which are either native or have been successfully introduced to our area. Successful landscaping, however, requires much more than just the selection of plants. Unless you have some experience, it is suggested than you employ the services of a trained or otherwise experienced landscaper, arborist, or forester. For best results it is recommended that careful consideration be made in purchasing the plants from the local nurseries (i.e. Cascade, McCall, and New Meadows). Plants brought in from the Treasure Valley may not survive our local weather conditions, microsites, and higher elevations. Timing can also be a serious consideration as the plants may have already broken dormancy and can be damaged by our late frosts. Appendix B SELECTED IDAHO NATIVE PLANTS SUITABLE FOR VALLEY COUNTY GROWING CONDITIONS Trees & Shrubs Acer circinatum (Vine Maple). Shrub or small tree 15-20' tall, Pacific Northwest native. Bright scarlet-orange fall foliage. Excellent ornamental. Alnus incana (Mountain Alder). A large shrub, useful for mid to high elevation riparian plantings. Good plant for stream bank shelter and stabilization. Nitrogen fixing root system. Alnus sinuata (Sitka Alder). A shrub, 6-1 5' tall. Grows well on moist slopes or stream banks. Excellent shrub for erosion control and riparian restoration. Nitrogen fixing root system. Amelanchier alnifolia (Serviceberry). One of the earlier shrubs to blossom out in the spring. -

Herbs, Spices and Flavourings Ebook, Epub
HERBS, SPICES AND FLAVOURINGS PDF, EPUB, EBOOK Tom Stobart | 240 pages | 14 Dec 2017 | Grub Street Publishing | 9781910690499 | English | London, United Kingdom Herbs, Spices and Flavourings PDF Book Free Sample.. The dried berries are slightly larger than peppercorns and impart a combination flavor of cinnamon, cloves, nutmeg, and pepper — hence the name allspice. For large batches of herbs and spices, a spice mill or a coffee grinder is convenient and quick. Leptotes bicolor Paraguay and southern Brazil Lesser calamint Calamintha nepeta , nipitella , nepitella Italy Licorice , liquorice Glycyrrhiza glabra Lime flower, linden flower Tilia spp. Mahleb is an aromatic spice ground from the internal kernel of the sour cherry pits of the mahleb cherry tree, Prunus mahaleb , native to Iran. Used instead of vinegar in salads and sauces when a milder acid is desired or when vinegar is objectionable. Culinary Australian Bangladeshi Indian Pakistani. The authors also focus on conventional and innovative analytical methods employed in this field and, last but not least, on toxicological, legal, and ethical aspects. Baharat is a blend of spices using allspice, black pepper, cardamom, cinnamon, cloves, coriander, cumin, nutmeg, and paprika — regional variations may also include loomi, mint, red chili peppers, rosebuds, saffron, and turmeric. Old Bay Seasoning. Twists, turns, red herrings, the usual suspects: These books have it all Often commercially blended with white and black peppercorns, pink peppercorns can be used to season any dish regular pepper would — although it should be noted that pink peppercorns are potentially toxic to small children. Avoid keeping herbs near the stove, in the refrigerator, or in the bathroom. -

Greek Recipes
Pita Bread Pan Size: Mixer w/ dough hook Yield: 4 Temperature: 500°F Portion Size: 1 Cooking Time: 20 minutes Ingredients Quantity Method 1/4 oz or 1 Dry yeast package Place water in mixer and add yeast. Let sit 5 minutes. Water 6 oz. 1 lb.-1 1/4 Bread Flour lbs. Add 1 lb. of flour, salt, oil, yogurt and Salt 1 Tbsp. cumin. Start on slow and mix until combined. If too wet, add more flour. Olive Oil 1 oz It should be a slightly stiff dough. Plain Yogurt 1/2 cup Knead in mixer until smooth and stretchy. Cumin 1/4 tsp. Cover in a lightly oiled bowl and let rest 60 minutes in a warm place. Divide into 4 pieces and round the pieces. Cover and let rest 10 minutes. Roll out into oblong pieces about a 1/4 " thick. Heat a medium saute pan or a cast iron griddle over medium high heat. When smoking, place one piece of dough on the pan and cook 30-40 seconds. Flip over and cook another minute when bubbles start to form flip over again. Brown slightly on both sides. Remove when puffs. Repeat. Hold in a towel or covered dish to keep warm. 1 Flatbread with Tomatoes and Olives (Laganes) Pan Size: Saute pan, sheet pan or pizza pan Yield: 2 Temperature: 500°F Baking Time: 8-10 minutes Ingredients Quantity Method Onion, thinly sliced 1 Heat a saute pan, add oil and onions. Lower heat and sweat until soft. Turn heat up and cook until Olive oil 1 Tbsp. -

Sumac Rhus Coriaria Tips for Use
Recipes Sumac Rhus coriaria Tips for Use: • The flavor is earthy, citrus and a bit sour • Make a marinade or salad dressing • Add to yogurt or sprinkle on hummus or even sweet potato fries • Add to your favorite meatloaf recipe • Use in making your own blend such as one of the many Za’atar recipes, which vary in different regions and from family to family. • Use Za’atar or other blends on chicken, lamb, fish or vegetables • Mix blends with olive oil for dipping bread • Top popcorn with sumac and salt for a salt and tart flavor Sumac Lemonade 1 cup water Lemon juice ¼ cup sumac syrup Ice Sumac Syrup: 1 cup water, 1 cup honey and 2 tablespoons ground sumac Make sumac syrup by combining water and honey in a small saucepan. Simmer over medium for 5 minutes then stir in sumac and infuse until cool, at least 10 minutes. Strain and store in an airtight container. Make sumac lemonade by combining water or sparkling water with sumac syrup, and a squeeze of lemon juice. Add ice and stir. Shape.com The Health Benefits of Sumac Spice Za’atar 1 tablespoon chopped fresh oregano 1 tablespoon sesame seeds 1 tablespoon sumac 1 teaspoon kosher salt 1 tablespoon ground cumin 1 teaspoon freshly ground black pepper Combine chopped fresh oregano, sumac, ground cumin and sesame seeds. Stir in kosher salt and freshly ground black pepper. Can be made 2 weeks ahead. Store airtight at room temperature. Silvena Rowe Bon appetite, www.bonappetit.com Za’atar Recipe: Middle Eastern Spice Mixure ¼ cup sumac 2 tablespoons marjoram 2 tablespoons thyme 2 tablespoons oregano 1 tablespoon roasted sesame seeds ©2018 by The Herb Society of America www.herbsociety.org 440-256-0514 9019 Kirtland Chardon Road, Kirtland, OH 44094 Recipes 1 teaspoon salt Grind the sesame seeds in a food processor or with a mortar and pestle. -

Five Flavor Profiles Fennel Salad with Feta, Pomegranate Seeds, and Sumac *
John Shaver JCC Winter Camp 2015 Cooking Together Today we learned how to balance flavors. We identified five distinct flavor profiles and discussed how they work together to create a delicious dish. Five Flavor Profiles FAT - Creamy, rich and luscious. Soft and silky on the tongue. Mellow and satisfying. - Ex. butter, olive oil, veg. oil, lard, cheese, nuts and seeds, avocados ACID - Sour, tart, and zesty. Sharp on the tongue. Perky and refreshing. - Ex. citrus fruits: lemon, lime, grapefruit; vinegars; cranberries; tomatoes SWEET - Sugary. Very pleasant on the tongue. Soothing and indulgent. - Ex. sugar; honey; fruits: pomegranates, strawberries, figs, bananas, apples AROMATICS - Impart character and quality of flavor. Provides the spirit and style of the dish. - Ex. herbs: parsley, cilantro, tarragon, basil, dill, thyme, bay leaf, rosemary, mint spices: pepper, cumin, coriander, cardamom, chilies, paprika, sumac vegetables: garlic, onions, carrots, celery, fennel, parsnips, asparagus SALT - Briny, earthy, pungent. Stinging on the tongue. Alluring and provocative. - Accentuates and focuses flavor. - Ex. sea salt, Kosher salt, Fleur de Sel, rock salt, Celtic sea salt, table salt Fennel salad with feta, pomegranate seeds, and sumac * We tasted each ingredient individually and discovered how they fit into the five flavor profiles. Fat: Lebanese extra virgin olive oil, Greek sheep’s milk feta cheese Acid: lemon juice Sweet: pomegranate seeds Aromatics: tarragon leaves, flat-leaf parsley, sumac, freshly ground black pepper Salt: Kosher salt Serves 4-6 For the dressing: 2-4 tbsp olive oil 1-2 tbsp lemon juice 1 tbsp sumac, start with half and add more to taste 4-6 tbsp tarragon leaves, whole 2-3 tbsp coarsely chopped flat-leaf parsley Salt, start with 1-2 teaspoons salt and add more to taste Freshly ground pepper, a twist or two 1. -

Spice Basics
SSpicepice BasicsBasics AAllspicellspice Allspice has a pleasantly warm, fragrant aroma. The name refl ects the pungent taste, which resembles a peppery compound of cloves, cinnamon and nutmeg or mace. Good with eggplant, most fruit, pumpkins and other squashes, sweet potatoes and other root vegetables. Combines well with chili, cloves, coriander, garlic, ginger, mace, mustard, pepper, rosemary and thyme. AAnisenise The aroma and taste of the seeds are sweet, licorice like, warm, and fruity, but Indian anise can have the same fragrant, sweet, licorice notes, with mild peppery undertones. The seeds are more subtly fl avored than fennel or star anise. Good with apples, chestnuts, fi gs, fi sh and seafood, nuts, pumpkin and root vegetables. Combines well with allspice, cardamom, cinnamon, cloves, cumin, fennel, garlic, nutmeg, pepper and star anise. BBasilasil Sweet basil has a complex sweet, spicy aroma with notes of clove and anise. The fl avor is warming, peppery and clove-like with underlying mint and anise tones. Essential to pesto and pistou. Good with corn, cream cheese, eggplant, eggs, lemon, mozzarella, cheese, olives, pasta, peas, pizza, potatoes, rice, tomatoes, white beans and zucchini. Combines well with capers, chives, cilantro, garlic, marjoram, oregano, mint, parsley, rosemary and thyme. BBayay LLeafeaf Bay has a sweet, balsamic aroma with notes of nutmeg and camphor and a cooling astringency. Fresh leaves are slightly bitter, but the bitterness fades if you keep them for a day or two. Fully dried leaves have a potent fl avor and are best when dried only recently. Good with beef, chestnuts, chicken, citrus fruits, fi sh, game, lamb, lentils, rice, tomatoes, white beans. -

Rhus Coriaria L.) and Thyme (Thymus Vulgaris
animals Article Growth, Carcass Composition, Haematology and Immunity of Broilers Supplemented with Sumac Berries (Rhus coriaria L.) and Thyme (Thymus vulgaris) Amir Ahmadian 1, Alireza Seidavi 1,* and Clive J. C. Phillips 2 1 Department of Animal Science, Rasht Branch, Islamic Azad University, Rasht 41335-3516, Iran; [email protected] 2 Centre for Animal Welfare and Ethics, School of Veterinary Science, University of Queensland, Gatton, QLD 4343, Australia; [email protected] * Correspondence: [email protected]; Tel.: +989-11-331-3073 Received: 26 January 2020; Accepted: 16 March 2020; Published: 19 March 2020 Simple Summary: Widespread use of antibiotics is known to cause resistance in bacteria, yet they are routinely used to improve growth performance in meat chickens. We investigated two medicinal plants, thyme and sumac berries, for their ability to function as an alternative to antibiotics in the diet of broilers. The actual plants or parts of the plants have rarely been fed before as supplements, mostly their extracted oils have been used instead. In our study, they were fed at 1–3% of the diet to investigate if the effects were dependent on the dose. We found evidence of improved immune systems with both medicinal plants when the chickens were tested for resistance to Newcastle Disease and influenza. Sumac berries, and to a lesser extent thyme, reduced the fat content of the birds’ abdomen, by 62 and 41%, respectively, reflecting an observed reduction in lipids in the blood. It is concluded that these two medicinal plants offer potential to replace antibiotics in the diet of meat chickens, as well as offering benefits in reducing the fat content of the birds. -
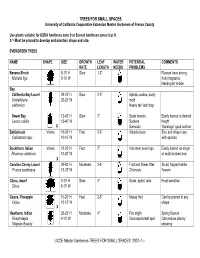
UCCE Master Gardeners TREES for SMALL SPACES 2007--1
TREES FOR SMALL SPACES University of California Cooperative Extension Master Gardeners of Fresno County Use plants suitable for USDA hardiness zone 9 or Sunset hardiness zones 8 or 9. X = Must be pruned to develop and maintain shape and size. EVERGREEN TREES NAME SHAPE SIZE GROWTH LEAF WATER POTENTIAL COMMENTS RATE LENGTH NEEDS PROBLEMS Banana Shrub 8-15’ H Slow 1-3” Flowers have strong Michelia figo 5-10’ W fruity fragrance Needs part shade Bay California Bay Laurel 20-25’ H Slow 2-5” Aphids, scales, sooty 20-25’ W mold Umbellularia californica Heavy fall leaf drop Sweet Bay 12-40’ H Slow 3” Scale insects Easily trained to desired Laurus nobilis 12-40’ W Suckers height X Sunscald ‘Saratoga” good cultivar Bottlebrush Varies 10-30’ H Fast 2-3” Attracts bees Size and shape vary Callistemon spp. 10-15’ W with species Buckthorn, Italian Varies 10-20’ H Fast 2” Volunteer seedlings Easily trained as single 10-20’ W or multi-trunked tree Rhamnus alaternus X Carolina Cherry Laurel 20-30’ H Moderate 2-4” Fruit and flower litter Small, fragrant white 15-25’ W Chlorosis flowers Prunus caroliniana Citrus, dwarf 8-10’ H Slow 3” Scale, aphid, ants Frost sensitive Citrus 8-10’ W Guava, Pineapple 15-20’ H Fast 2-3” Messy fruit Can be pruned to any Feijoa 10-15’ W shape X Hawthorn, Indian 20-25’ H Moderate 4” Fire blight Spring flowers Rhaphiolepis 8-10’ W Cercospora leaf spot Can reduce size by ‘Majestic Beauty’ shearing UCCE Master Gardeners TREES FOR SMALL SPACES 2007--1-- EVERGREEN TREES—Cont. -

And Soda Bread, Lescure Salted Butter
N i b b l e s S a l a d s • The Modern Pantry sourdough; poppy seed, • Grilled goats cheese, artichoke, roast sumac & quinoa lavosh; and soda bread, red onion, freekeh & sweetcorn salad, Lescure salted butter £3.50 spiced pumpkin seeds, cumin dressing £9.50 or £15.50 • Spiced nuts & seeds £3.50 • Cumin roast salsify, rhubarb, pickled carrot, • Persian spiced cashews/ Liquorice macadamias/ Marinated olives £4.00 fried okra & baby spinach salad, sumac potato crisps, garlic membrillo dressing £8.50 or £15.00 M a i n s Small plates & S t a r t e r s • Cauliflower, parsnip & king oyster mushroom • Cauliflower laksa, lemon soup & black tahini stew, truffled white polenta, curried tofu yoghurt, puffed wild rice £6.90 crisps £16.80 • Roast beetroot, hijiki & feta fritters, black • Cumin roast Cornish fish, sesame grilled garlic & hazelnut yoghurt, lemon salsa £7.40 leeks, miso onion broth, red chilli & • Cumin roast lentil patty, swede, carrot, turnip coriander salad £21.50 & mange tout salad, tonkatsu dressing, • Slow roast Gloucester Old Spot pork belly, beetroot aioli, spiced peanut crumb £8.80 red cabbage puree, spiced parsnip, feijoa & • Roast venison haunch, pistachio puree, apple relish £18.00 lemon, manuka honey & mustard seed dressing £8.50 • Tandoori spiced duck breast, pickled butternut squash, chervil root & shiitake • Beetroot, carrot & chickpea hash, green mushrooms, Greek youghurt dressing £21.50 romesco sauce, grilled tenderstem broccoli, aubergine relish £7.50 • Fenugreek & paprika roast lamb rump, tomato, aubergine puree, curry -

Landscape Plant Palette
City of Aliso Viejo July 6, 2005 Updated February 8, 2007 LANDSCAPE PLANT PALETTE The following listing of plant materials comprise the City of Aliso Viejo’s plant palette of low water plants and is to be used for all public and private improvement projects within the City. The City Engineer and the Planning Director have the discretion to discuss, review, and approve alternate plant materials on a project by project basis and accept other materials which are considered low water drought tolerant. Trees Botanical Name Common Name Agonis flexuosa Australian Myrtle Albizia julibrissin Silk Tree Arbutus ‘Marina’ N.C.N. Brachychiton discolor Queensland Lacebark Brachychiton populneus Bottle Tree Cassia leptophylla Gold Medallion Tree Cercis occidentalis Western Redbud Chitalpa tashkentensis Chitalpa Cinnamomum camphora Camphor Tree Geijera parvifolia Australian Willow Koelreuteria bipinnatta Chinese Flame Tree Koelreuteria paniculata Goldenrain Tree Laurus nobilis Sweet Bay Leptospermum laevigatum Australian Tea Tree Liquidambar styraciflua Sweet Gum Metrosideros excelsus New Zealand Christmas Tree Olea europae ‘Wilsonii’ Fruitless Olive Parkinsonia aculeate Mexican Palo Verde Pinus eldarica Afghan Pine Pinus halepensis Aleppo Pine Pinus pinea Italian Stone Pine Pinus spp Pine Platanus acerifolia London Plane Tree Prosopis chilensis Mesquite Prosopis chilensis ‘Thornless’ Chilean Mesquite Prosopis velutina Arizona Mesquite Rhus lancea African Sumac Quercus agrifolia Coast Live Oak Quercus engelmannii Engelmann Oak Quercus ilex Holly Oak Quercus -

Rhus Glabra) Staghorn Sumac (Rhus Typhina
Weed Identification and Control Sheet: www.goodoak.com/weeds Smooth sumac (Rhus glabra) Staghorn sumac (Rhus typhina) DESCRIPTION: Sumac is native throughout eastern U.S. and southern Canada but occurs most commonly in east- ern U.S. on forest edges, abandoned fields and roadsides. Their interesting branching patterns, height, bright-red fall foliage and colony forming habit make these woody perennial shrubs of the cashew family an attractive option for screening in a landsccape setting. The bright red fruit clusters are an important winter food resource for many bird species and small mammals. Although both smooth and staghorn sumac are beneficial native shrubs, they can rap- idly form massive thickets in open woodlands, savannas and prairie settings, making them a target for control in these endangered ecocystems. The shade produced by sumac colonies can suppress native groundlayer vegetation and tree seedling germination. IDENTIFICATION: Both sumac species tend to form broad, spreading colonies. Smooth sumac tends to be wider than it is tall. Leaves are pinnately compound, lance-shaped and saw-toothed, with 7 to 31 leaflets. Leaves turn bright red to orange color in the fall. Both sumac species have stout branches that excude a milky sap when cut. Yellowish green flower clusters develop from late May into August. Fruit is a bright red spike containing many berrylike drupes. Smooth sumac twigs are nearly hairless, often covered with a whitish ‘bloom’ that can be ribbed off. Twigs of the staghorn sumac are velvety. The aboveground portion of the plant is relatively short-lived but roots persist and form new stems. -

A Review on Mycotoxins and Microfungi in Spices in the Light of the Last Five Years
toxins Review A Review on Mycotoxins and Microfungi in Spices in the Light of the Last Five Years Darina Pickova 1,* , Vladimir Ostry 1,2 , Jan Malir 3, Jakub Toman 1 and Frantisek Malir 1 1 Department of Biology, Faculty of Science, University of Hradec Kralove, Rokitanskeho 62, CZ-50003 Hradec Kralove, Czech Republic; [email protected] (V.O.); [email protected] (J.T.); [email protected] (F.M.) 2 Center for Health, Nutrition and Food in Brno, National Institute of Public Health in Prague, Palackeho 3a, CZ-61242 Brno, Czech Republic 3 Department of Public Law, Institute of State and Law, Czech Academy of Sciences, Narodni 18, CZ-11600 Prague, Czech Republic; [email protected] * Correspondence: [email protected]; Tel.: +420-722-049-025 Received: 11 November 2020; Accepted: 9 December 2020; Published: 11 December 2020 Abstract: Spices are imported worldwide mainly from developing countries with tropical and/or subtropical climate. Local conditions, such as high temperature, heavy rainfall, and humidity, promote fungal growth leading to increased occurrence of mycotoxins in spices. Moreover, the lack of good agricultural practice (GAP), good manufacturing practice (GMP), and good hygienic practice (GHP) in developing countries are of great concern. This review summarizes recent data from a total of 56 original papers dealing with mycotoxins and microfungi in various spices in the last five years. A total of 38 kinds of spices, 17 mycotoxins, and 14 microfungi are discussed in the review. Worldwide, spices are rather overlooked in terms of mycotoxin regulations, which usually only cover aflatoxins (AFs) and ochratoxin A (OTA).