1 Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report of the Workshop on Eel and Salmon DCF Data (WKESDCF)
ICES WKESDCF REPORT 2012 ICES ADVISORY COMMITTEE ICES CM / ACOM:62 REF. PGCCDBS, WGEEL, WGNAS, WGRECORDS Report of the Workshop on Eel and Salmon DCF Data (WKESDCF) 3 – 6 July 2012 ICES HQ, Copenhagen, Denmark International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer H. C. Andersens Boulevard 44–46 DK-1553 Copenhagen V Denmark Telephone (+45) 33 38 67 00 Telefax (+45) 33 93 42 15 www.ices.dk [email protected] Recommended format for purposes of citation: ICES. 2012. Report of the Workshop on Eel and Salmon DCF Data (WKESDCF), 3 – 6 July 2012, ICES HQ, Copenhagen, Denmark. ICES CM / ACOM:62. 67 pp. For permission to reproduce material from this publication, please apply to the Gen- eral Secretary. The document is a report of an Expert Group under the auspices of the International Council for the Exploration of the Sea and does not necessarily represent the views of the Council. © 2012 International Council for the Exploration of the Sea ICES WKESDCF REPORT 2012 | i Contents 1 Introduction .................................................................................................................... 3 1.1 Purpose of the Workshop .................................................................................... 3 1.2 Organization of the meeting ............................................................................... 3 1.3 Structure of the report .......................................................................................... 4 2 Current DCF requirements relating to eel and salmon -

RECOVERY POTENTIAL ASSESSMENT of AMERICAN EEL (Anguilla Rostrata) in EASTERN CANADA
Gulf, Central & Arctic, Maritimes, Canadian Science Advisory Secretariat Newfoundland and Labrador, Quebec Science Advisory Report 2013/078 RECOVERY POTENTIAL ASSESSMENT OF AMERICAN EEL (Anguilla rostrata) IN EASTERN CANADA Illustration credit: US Fish and Wildlife Service Figure 1. American Eel Recovery Potential Assessment zones in eastern Canada. Interior zones boundaries follow watershed limits and exterior boundaries follow the 500 m bathymetric contour. Context: The American Eel (Anguilla rostrata) is widely distributed in freshwater, estuarine and protected coastal areas of eastern Canada (including Lake Ontario and the upper St. Lawrence River). It is a facultatively catadromous fish, spawning in the Sargasso Sea and the recruiting stages return to continental waters along the western North Atlantic Ocean to grow and mature. In April 2012, the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) concluded that American Eel from eastern Canada belonged to one Designatable Unit, and assessed its status as Threatened because of declines in abundance indices over the last two or more generations. American Eel is being considered for legal listing under the Species at Risk Act (SARA; the Act). In advance of making a listing decision, Fisheries and Oceans Canada (DFO) has been asked to undertake a Recovery Potential Assessment (RPA). This RPA summarizes the current understanding of the distribution, abundance and population trends of American Eel, along with recovery targets and timeframes. The current state of knowledge about habitat requirements, threats to both habitat and American Eel, and measures to mitigate these impacts are also included. This information may be used to inform both scientific and socio-economic elements of the listing decision, development of a recovery strategy and action plan, and to support decision-making with regards to the issuance of permits, agreements and related conditions of the Act. -

Lake Ontario Fish Communities and Fisheries
LAKE ONTARIO FISH COMMUNITIES AND FISHERIES: 2013 ANNUAL REPORT OF THE LAKE ONTARIO MANAGEMENT UNIT LAKE ONTARIO FISH COMMUNITIES AND FISHERIES: 2013 ANNUAL REPORT OF THE LAKE ONTARIO MANAGEMENT UNIT Prepared for the Great Lakes Fishery Commission 2014 Lake Committee Meetings Windsor, ON Canada March 24-28, 2014 © 2014, Queen’s Printer for Ontario Printed in Picton, Ontario, Canada March 2014 Report ISSN 1201-8449 Please cite this report as follows: Ontario Ministry of Natural Resources. 2014. Lake Ontario Fish Communities and Fisheries: 2013 Annual Report of the Lake Ontario Management Unit. Ontario Ministry of Natural Resources, Picton, Ontario, Canada. Report available on the following website: http://www.glfc.org/lakecom/loc/mgmt_unit/index.html TABLE OF CONTENTS Foreword ............................................................................................................................................. v 1. Status of Fish Communities 1.1 Nearshore Fish Community .................................................................................................. 1 1.2 Offshore Pelagic Fish Community ....................................................................................... 1 1.3 Offshore Benthic Fish Community ...................................................................................... 2 2. Index Fishing Projects 2.1 Ganaraska Fishway Rainbow Trout Assessment .................................................................. 3 2.2 Eastern Lake Ontario and Bay of Quinte Fish Community Index Gill Netting -

Spatial Analysis of American Eel (Anguilla Rostrata)
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.17.995183; this version posted March 17, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. This article is a US Government work. It is not subject to copyright under 17 USC 105 and is also made available for use under a CC0 license. 1 2 3 4 Spatial analysis of American Eel (Anguilla rostrata), fish passage and land use in Chesapeake 5 Bay tributaries 6 7 Walker, Nicholas J.1*; Prasad, V.1; de Mutsert, K.1; Dolloff, C.A.2; Aguirre, A.A.1 8 9 1 Department of Environmental Science & Public Policy, George Mason University, Fairfax, 10 Virginia, 22030, USA 11 12 2 USDA Forest Service, Southern Research Station, Fish and Wildlife Conservation, Virginia Tech 13 University, Blacksburg, Virginia, 24061, USA 14 15 * Corresponding author 16 E-mail: [email protected] 17 18 ¶ These authors contributed equally to this work. 19 20 Abstract 21 Catadromous eels are found in more habitats than any other fish and are capable of inhabiting 22 marine, brackish and freshwater environments. In this study we used the American Eel (Anguilla 23 rostrata) as a bioindicator organism to create a novel method of using spatial analysis to study 24 species conservation over landscape scales. We built a model of the subwatersheds of the 25 Chesapeake Bay using a Digital Elevation Model (DEM) and overlaid eel density data (> 1 million 26 eels sampled), dam density data and land use in ArcGIS. Dam construction in the study area peaked 27 between 1955 and 1975, possibly as a result of flood control measures. -

American Eel Anguilla Rostrata
COSEWIC Assessment and Status Report on the American Eel Anguilla rostrata in Canada SPECIAL CONCERN 2006 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2006. COSEWIC assessment and status report on the American eel Anguilla rostrata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 71 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Production note: COSEWIC would like to acknowledge V. Tremblay, D.K. Cairns, F. Caron, J.M. Casselman, and N.E. Mandrak for writing the status report on the American eel Anguilla rostrata in Canada, overseen and edited by Robert Campbell, Co-chair (Freshwater Fishes) COSEWIC Freshwater Fishes Species Specialist Subcommittee. Funding for this report was provided by Environment Canada. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Évaluation et Rapport de situation du COSEPAC sur l’anguille d'Amérique (Anguilla rostrata) au Canada. Cover illustration: American eel — (Lesueur 1817). From Scott and Crossman (1973) by permission. ©Her Majesty the Queen in Right of Canada 2004 Catalogue No. CW69-14/458-2006E-PDF ISBN 0-662-43225-8 Recycled paper COSEWIC Assessment Summary Assessment Summary – April 2006 Common name American eel Scientific name Anguilla rostrata Status Special Concern Reason for designation Indicators of the status of the total Canadian component of this species are not available. -

Justification For, and Status Of, American Eel Elver Fisheries in Scotia-Fundy Region
Not to be cited without Ne pas citer sans permission of the authors' autorisation des auteurs ' DFO Atlantic Fisheries MPO Pêches de l'Atlantique Research Document 95/2 Document de recherche 95/2 Justification for, and status of, American eel elver fisheries in Scotia-Fundy Region by B.M. Jessop Biological Sciences Branc h Department of Fisheries and Oceans P.O. Box 550 Halifax, Nova Scotia B3J 2S7 'This series documents the scientific basis for the 'La présente série documente les bases scientifiques evaluation of fisheries resources in Atlantic Canada. des évaluations des ressources halieutiques sur la côte As such, it addresses the issues of the day in the time atlantique du Canada. Elle traite des problèmes frames required and the documents it contains are not courants selon les échéanciers dictés . Les documents intended as definitive statements on the subjects qu'elle contient ne doivent pas être considérés comme addressed but rather as progress reports on ongoing des énoncés définitifs sur les sujets traités, mais plutôt investigations . comme des rapports d'étape sur les études en cours . Research documents are produced in the official Les Documents de recherche sont publiés dans la language in which they are provided to the secretariat . langue officielle utilisée dans le manuscrit envoyé au secrétariat . 2 Abstract North Ame rica contributes less th an 1% to the total world production of eels (AnQuillidae) for human consumption . Elvers of the native species have been fished by m any coastal nations of Europe An illa anguilla) since the e arly 1900s, by Japan L . 'a nica since the 1920s, and in various U .S. -
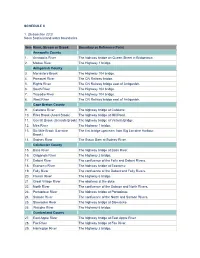
Nova Scotia Inland Water Boundaries Item River, Stream Or Brook
SCHEDULE II 1. (Subsection 2(1)) Nova Scotia inland water boundaries Item River, Stream or Brook Boundary or Reference Point Annapolis County 1. Annapolis River The highway bridge on Queen Street in Bridgetown. 2. Moose River The Highway 1 bridge. Antigonish County 3. Monastery Brook The Highway 104 bridge. 4. Pomquet River The CN Railway bridge. 5. Rights River The CN Railway bridge east of Antigonish. 6. South River The Highway 104 bridge. 7. Tracadie River The Highway 104 bridge. 8. West River The CN Railway bridge east of Antigonish. Cape Breton County 9. Catalone River The highway bridge at Catalone. 10. Fifes Brook (Aconi Brook) The highway bridge at Mill Pond. 11. Gerratt Brook (Gerards Brook) The highway bridge at Victoria Bridge. 12. Mira River The Highway 1 bridge. 13. Six Mile Brook (Lorraine The first bridge upstream from Big Lorraine Harbour. Brook) 14. Sydney River The Sysco Dam at Sydney River. Colchester County 15. Bass River The highway bridge at Bass River. 16. Chiganois River The Highway 2 bridge. 17. Debert River The confluence of the Folly and Debert Rivers. 18. Economy River The highway bridge at Economy. 19. Folly River The confluence of the Debert and Folly Rivers. 20. French River The Highway 6 bridge. 21. Great Village River The aboiteau at the dyke. 22. North River The confluence of the Salmon and North Rivers. 23. Portapique River The highway bridge at Portapique. 24. Salmon River The confluence of the North and Salmon Rivers. 25. Stewiacke River The highway bridge at Stewiacke. 26. Waughs River The Highway 6 bridge. -

Hudson River National Estuarine Research Reserve Management Plan OCTOBER 1, 2019–OCTOBER 1, 2024
Hudson River National Estuarine Research Reserve Management Plan OCTOBER 1, 2019–OCTOBER 1, 2024 Andrew M. Cuomo, Governor | Basil Seggos, Commissioner Acknowledgments This plan was prepared by staff of the Hudson River National Estuarine Research Reserve, including Betsy Blair, Chris Bowser, Ann-Marie Caprioli, Brian DeGasperis, Sarah Fernald, Heather Gierloff, Emilie Hauser, Dan Miller, and Sarah Mount, with the assistance of Andy Burgher, Cathy Kittle, and Bill Rudge in the New York State Department of Environmental Conservation; Ed McGowan of the New York State Office of Parks, Recreation and Historic Preservation; and Nina Garfield and Ann Weaver of the National Oceanic and Atmospheric Administration, Office for Coastal Management. We appreciate input that has informed development of this plan provided by other colleagues, local leaders, county officials, environmental organizations, researchers, educators, and marsh managers. Suggested citation: New York State Department of Environmental Conservation (NYSDEC). 2019. Hudson River National Estuarine Research Reserve Management Plan. Albany, NY. Table of Contents Executive Summary ................................................................................................................................... iv Introduction ................................................................................................................................................. 1 The Reserve ......................................................................................................................................... -

South Western Nova Scotia
Netukulimk of Aquatic Natural Life “The N.C.N.S. Netukulimkewe’l Commission is the Natural Life Management Authority for the Large Community of Mi’kmaq /Aboriginal Peoples who continue to reside on Traditional Mi’Kmaq Territory in Nova Scotia undisplaced to Indian Act Reserves” P.O. Box 1320, Truro, N.S., B2N 5N2 Tel: 902-895-7050 Toll Free: 1-877-565-1752 2 Netukulimk of Aquatic Natural Life N.C.N.S. Netukulimkewe’l Commission Table of Contents: Page(s) The 1986 Proclamation by our late Mi’kmaq Grand Chief 4 The 1994 Commendation to all A.T.R.A. Netukli’tite’wk (Harvesters) 5 A Message From the N.C.N.S. Netukulimkewe’l Commission 6 Our Collective Rights Proclamation 7 A.T.R.A. Netukli’tite’wk (Harvester) Duties and Responsibilities 8-12 SCHEDULE I Responsible Netukulimkewe’l (Harvesting) Methods and Equipment 16 Dangers of Illegal Harvesting- Enjoy Safe Shellfish 17-19 Anglers Guide to Fishes Of Nova Scotia 20-21 SCHEDULE II Specific Species Exceptions 22 Mntmu’k, Saqskale’s, E’s and Nkata’laq (Oysters, Scallops, Clams and Mussels) 22 Maqtewe’kji’ka’w (Small Mouth Black Bass) 23 Elapaqnte’mat Ji’ka’w (Striped Bass) 24 Atoqwa’su (Trout), all types 25 Landlocked Plamu (Landlocked Salmon) 26 WenjiWape’k Mime’j (Atlantic Whitefish) 26 Lake Whitefish 26 Jakej (Lobster) 27 Other Species 33 Atlantic Plamu (Salmon) 34 Atlantic Plamu (Salmon) Netukulimk (Harvest) Zones, Seasons and Recommended Netukulimk (Harvest) Amounts: 55 SCHEDULE III Winter Lake Netukulimkewe’l (Harvesting) 56-62 Fishing and Water Safety 63 Protecting Our Community’s Aboriginal and Treaty Rights-Community 66-70 Dispositions and Appeals Regional Netukulimkewe’l Advisory Councils (R.N.A.C.’s) 74-75 Description of the 2018 N.C.N.S. -

Manual for Provision of Upstream Migration Facilities for Eel and Elver
w w w.environment-agency.gov.uk Manual for provision of upstream migration facilities for Eel and Elver Science Report SC020075/SR2 The Environment Agency is the leading public body protecting and improving the environment in England and Wales. It’s our job to make sure that air, land and water are looked after by everyone in today’s society, so that tomorrow’s generations inherit a cleaner, healthier world. Our work includes tackling flooding and pollution incidents, reducing industry’s impacts on the environment, cleaning up rivers, coastal waters and contaminated land, and improving wildlife habitats. AUTHORS: PUBLISHED BY: David J.Solomon, Michael H.Beach Environment Agency, Rio House, Waterside Drive, Aztec West, Almondsbury, Bristol, BS32 4UD Dissemination Status: Tel: 01454 624400 Fax: 01454 624409 Internal: Released to Regions External Public Domain ISBN: 1844323498 Statement of Use: © Environment Agency December 2004 This manual sets out design criteria for upstream passage facilities at obstructions for eels and elvers, based upon assessments of the All rights reserved. This document may be reproduced with prior migratory behaviour and ecology of Anguilla spp. It is intended for permission of the Environment Agency. use by fisheries managers with responsibility for or an interest in the management of stocks of eels and elvers. It is largely based upon This report is printed on Cyclus Print, a 100% recycled stock, the R&D Technical Report W2-070/TR1 “Fish pass design for eel which is 100% post consumer waste and is totally chlorine free. and elver (Anguilla anguilla)”. Water used is treated and in most cases returned to source in better condition than removed. -

Black Bear Hydro Partners, LLC Orono Project (FERC No. 2710)
November 12, 2020 Orono Project (FERC No. 2710) Stillwater Project (FERC No. 2712) Ms. Shannon Ames, Executive Director Low Impact Hydropower Institute 329 Massachusetts Avenue, Suite 2 Lexington, MA 02420 Subject: Low Impact Hydropower Institute Application for the Orono Project (FERC No. 2710) and Stillwater Project (FERC No. 2712) Dear Ms. Ames: On behalf of Black Bear Hydro Partners, LLC, Black Bear Development Holdings, LLC, and Black Bear SO, LLC (collectively, “Black Bear”), owners and licensees of the Orono and Stillwater Hydroelectric Projects (“Projects”) (FERC Nos. 2710 and 2712, respectively) and affiliates of Brookfield Renewable, please find attached a revised application for recertification of the Projects, which are located on the Stillwater Branch of the Penobscot River in Maine. Black Bear is requesting recertification of these facilities, which are currently certified through November 30, 2020 per LIHI correspondence dated June 1, 2020. Black Bear submitted an initial certification application to the Low Impact Hydropower Institute (LIHI) on July 6, 2020. LIHI completed the initial Intake Review on August 5, 2020. The current application includes the following required submittals as revised in response to the LIHI Intake Review: • Introduction • Project Description and LIHI Table B-1 • Zones of Effect descriptions and overview maps and images • Matrix of Alternative Standards for each Zone of Effect identified evaluating the LIHI certification standards for each requisite criterion, including water quality, fish passage and recreation • Sworn Statement and Waiver Form • Facility Contacts Form including pertinent NGOs, as appropriate. • List of hyperlinks and supplemental documentation for pertinent FERC and regulatory documents for the Projects Please call me at (207) 755-5606 or email me at [email protected] if you have any questions or need additional information regarding this submittal. -

Compiled Eel Abstracts: American Fisheries Society 2014 Annual Meeting August 18-21, 2014 Quebec City, Quebec, Canada [Compiled/Organized by R
Compiled Eel Abstracts: American Fisheries Society 2014 Annual Meeting August 18-21, 2014 Quebec City, Quebec, Canada [Compiled/organized by R. Wilson Laney, U.S. Fish and Wildlife Service, Raleigh, NC, USA] International Eel Symposium 2014: Are Eels Climbing Back up the Slippery Slope? Monday, August 18, 2014: 1:30 PM 206B (Centre des congrès de Québec // Québec City Convention Centre) Martin Castonguay, Institut Maurice-Lamontagne, Pêches et Océans Canada, Mont-Joli, QC, Canada David Cairns, Science Branch, Fisheries and Oceans Canada, Charlottetown, PE, Canada Guy Verreault, Ministere du Développement durable, de l'Environnement, de la Faune et des Parcs, Riviere-du-Loup, QC, Canada John Casselman, Dept. of Biology, Queen's University, Kingston, ON, Canada This talk will introduce the symposium. The first part will outline how the Symposium is structured, where to see Symposium posters, the panel, publication plans, etc. This would be followed by a brief outline of eel science and conservation issues, and how the Symposium will address these. I will then present with some level of detail the themes that the symposium will cover. The Introduction would also state the question that will be addressed by the panel discussion, and ask participants to keep this question in mind as they listen to the various talks. In closing, the introductory talk will outline the purpose of the Canada/USA Eel governance session that will be held at the very end of the meeting, after the Symposium panel. Publishing in the eel symposium proceedings - An orientation from the Editor-in- Chief of the ICES Journal of Marine Science Monday, August 18, 2014: 5:00 PM 206B (Centre des congrès de Québec // Québec City Convention Centre) Howard Browman, Marine Ecosystem Acoustics, Institute of Marine Research, Storebø, Norway I will provide symposium participants with a status report on the ICES Journal of Marine science.