<I>Anigozanthos Manglesii</I>
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beijing Olympic Mascots
LEVEL – Lower primary FLORAL EMBLEMS DESCRIPTION In these activities, students learn about the floral emblems of Great Britain. They discuss their own responses to the emblems and explore design elements and features including colours, shapes, lines and their purpose before colouring a picture. These cross-curriculum activities contribute to the achievement of the following: Creative and visual arts • Selects, combines and manipulates images, shapes and forms using a range of skills, techniques and processes. English • Interprets and discusses some relationships between ideas, information and events in visual texts for general viewing. SUGGESTED TIME approximately 15-30 minutes for each activity (this may be customised accordingly) WHAT YOU NEED • photographs or actual samples of the floral emblem for your state or territory http://www.anbg.gov.au/emblems/index.html • photographs or actual samples of the floral emblems of Great Britain – Rose (England), Shamrock (Ireland), Thistle (Scotland) and Daffodil (Wales) o http://www.flickr.com/groups/roses/ o http://www.flickr.com/photos/tags/shamrock/clusters/green-irish-stpatricksday/ o http://www.flickr.com/search/?q=Thistle+ o http://www.flickr.com/groups/daffodilworld/ • copies of Student handout • paint, brushes, markers, crayons, glitter and other art materials ACTIVITIES The following activities may be completed independently or combined as part of a more comprehensive learning sequence, lesson or educational program. Please refer to your own state or territory syllabus for more explicit guidelines. Australia’s floral emblems 1. Show the class a picture or sample of Golden Wattle, along with the floral emblem for your state or territory. Ask the class if anyone has these flowers growing in their garden or local area. -

A Potential New Cutflower for Australia - Haemodorum Coccineum
A Potential New Cutflower For Australia - Haemodorum Coccineum A report for the Rural Industries Research and Development Corporation by Margaret Johnston and Alenna McMah September 2006 RIRDC Publication No 06/087 RIRDC Project No UQ-117A © 2006 Rural Industries Research and Development Corporation. All rights reserved. ISBN 1 74151 350 2 ISSN 1440-6845 Haemodorum coccineum production in south-east Queensland Publication No. 06/087 Project No. UQ117A The information contained in this publication is intended for general use to assist public knowledge and discussion and to help improve the development of sustainable industries. The information should not be relied upon for the purpose of a particular matter. Specialist and/or appropriate legal advice should be obtained before any action or decision is taken on the basis of any material in this document. The Commonwealth of Australia, Rural Industries Research and Development Corporation, the authors or contributors do not assume liability of any kind whatsoever resulting from any person's use or reliance upon the content of this document. This publication is copyright. However, RIRDC encourages wide dissemination of its research, providing the Corporation is clearly acknowledged. For any other enquiries concerning reproduction, contact the Publications Manager on phone 02 6272 3186. Researcher Contact Details Dr Margaret Johnston Mrs Alenna McMah Centre for Native Floriculture Boomajarril Native Flower Farm Phone: 07 54601240 Phone: 07 54665668 Fax: 07 54601112 Fax: 07 54665668 Email: [email protected] Email: [email protected] In submitting this report, the researcher has agreed to RIRDC publishing this material in its edited form. RIRDC Contact Details Rural Industries Research and Development Corporation Level 2, 15 National Circuit BARTON ACT 2600 PO Box 4776 KINGSTON ACT 2604 Phone: 02 6272 4819 Fax: 02 6272 5877 Email: [email protected]. -

WRA Species Report
Family: Haemodoraceae Taxon: Anigozanthos flavidus Synonym: NA Common Name: Evergreen kangaroo paw Tall kangaroo paw Questionaire : current 20090513 Assessor: Chuck Chimera Designation: H(HPWRA) Status: Assessor Approved Data Entry Person: Chuck Chimera WRA Score 12 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- Intermediate substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 n 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 n 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see y Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see y Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see Appendix 2) 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic y=1, n=0 403 Parasitic y=1, n=0 n 404 Unpalatable to grazing animals y=1, n=-1 405 Toxic to animals y=1, n=0 n 406 Host for -

Tranen Seed Species Text
Tranen Pty Ltd, 1/110 Jersey Street, Jolimont, WA, 6014 p (08) 9284 1399 f 9284 1377 [email protected] ABN 37 054 506 446 ACN 054 506 446 NATIVE SEED SALES Tranen specialises in the supply of native seeds of plant species indigenous to Western Australia. Our clients base is very diverse, and includes landscapers, developers, nurseries, land care groups, government departments, mining and construction companies, farmers, researchers and schools. SEED PRICES Seed prices vary a lot between species, and generally reflect the availability and the degree of difficulty in harvesting and processing the seed. Seasonal conditions, availability and demand can have significant effects on market prices in the short term. Please contact us for pricing and availability, preferably by email if your species list is large, or call us if you prefer. Quotations will remain valid for 30 days, but availability will be subject to prior sale. SPECIES LIST A list of species that we usually stock follows. Species names are those current in Florabase. Names that have recently changed are shown in brackets. If you are unable to find a species in our list, please contact us to check if the name has been changed. Please do not hesitate to enquire about southwestern WA native species that you may require which are not listed in our list, and we will be pleased to endeavour to source them for you. Please feel free to contact us if you require further technical information, including information on seed counts for particular species CONDITIONS OF SALE Prices All prices we quote are in Australian dollars. -

Vegetative Anatomy of the Haemodoraceae and Its Phylogenetic Significance
Int. J. Plant Sci. 178(2):117–156. 2017. q 2016 by The University of Chicago. All rights reserved. 1058-5893/2017/17802-0004$15.00 DOI: 10.1086/689199 VEGETATIVE ANATOMY OF THE HAEMODORACEAE AND ITS PHYLOGENETIC SIGNIFICANCE Layla Aerne-Hains1,* and Michael G. Simpson2,* *Department of Biology, San Diego State University, San Diego, California 92182, USA Editor: Félix Forest Premise of research. Haemodoraceae are a relatively small monocot family consisting of 14 genera and approximately 108 species and are distributed in parts of Australia, southern Africa, South and Central America, and eastern North America. The family is divided into two subfamilies, Haemodoroideae and Conostylidoideae. This research focuses on the vegetative anatomy of the family, with an emphasis on leaf anatomical features. The aims of this project are (1) to acquire new vegetative anatomical data for a large selection of Haemodoraceae and (2) to evaluate these data in the context of both phylogenetic relationships and environmental factors. Methodology. Cross sections of roots, stems (scapes), or leaves of 60 species and 63 ranked taxa from all 14 genera of the family were prepared and stained using standard histological methods, and SEMs were made of the leaf surface. Line drawings were prepared of leaf cross sections of an exemplar of each genus. Tissues and cells were examined and photographed, and comparisons were made among taxa. For leaf epidermal cells, the ratio of cell wall transectional area∶cell transectional area was calculated and plotted. Several dis- crete anatomical characters and character states were defined and plotted on a recently derived cladogram and examined for phylogenetic signal. -

A Molecular Phylogenetic Study of Generic and Subgeneric Relationships in the Southwest Australian Endemics Conostylis and Blancoa (Haemodoraceae) Stephen D
Aliso: A Journal of Systematic and Evolutionary Botany Volume 22 | Issue 1 Article 41 2006 A Molecular Phylogenetic Study of Generic and Subgeneric Relationships in the Southwest Australian Endemics Conostylis and Blancoa (Haemodoraceae) Stephen D. Hopper The University of Western Australia Mark W. Chase Royal Botanic Gardens, Kew Michael F. Fay Royal Botanic Gardens, Kew Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Hopper, Stephen D.; Chase, Mark W.; and Fay, Michael F. (2006) "A Molecular Phylogenetic Study of Generic and Subgeneric Relationships in the Southwest Australian Endemics Conostylis and Blancoa (Haemodoraceae)," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 22: Iss. 1, Article 41. Available at: http://scholarship.claremont.edu/aliso/vol22/iss1/41 Aliso 22, pp. 527-538 © 2006, Rancho Santa Ana Botanic Garden A MOLECULAR PHYLOGENETIC STUDY OF GENERIC AND SUBGENERIC RELATIONSHIPS IN THE SOUTHWEST AUSTRALIAN ENDEMICS CONOSTYLIS AND BLANCOA (HAEMODORACEAE) 3 2 STEPHEN D. HOPPER, 1• MARK W. CHASE, AND MICHAEL F. FAY2 1School of Plant Biology, Faculty of Natural and Agricultural Sciences, The University of Western Australia, Crawley, Western Australia 6009, Australia; 2Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3DS, UK 3Corresponding author ( [email protected]) ABSTRACT We sequenced the plastid gene matK and the nuclear ribosomal spacer ITS for 39 of the 47+ species of Conostylis as well as its monotypic sister genus Blancoa, which some authors have included within Conostylis. Conostylis received 99% bootstrap support as monophyletic, with 100% support that Blancoa is its sister. Within Conostylis, the study provides strong support for two large sister clades, which we refer to as clades A (100%) and B (99%). -

Abstracts 2009.Pdf
AUSTRALASIAN PLANT PATHOLOGY SOCIETY, WA STUDENT SYMPOSIUM ABSTRACTS 23 OCTOBER 2009, DEC, KENSINGTON 1. Mycotoxic metabolites produced by Fusarium species associated with feed refusal disorders in Western Australia Diana Tan 1, E.L. Ghisalberti 2, G.R. Flematti 2, M.J. Barbetti 1,3 and K. Sivasithamparam 1 1 School of Plant Biology, Faculty of Natural and Agricultural Sciences, The University of Western Australia, Crawley 6009 2 School of Biomedical, Biomolecular and Chemical Sciences, Faculty of Life and Physical Sciences, The University of Western Australia, Crawley 6009 3 Department of Agriculture and Food Western Australia, South Perth. 6151 Email: [email protected] Sheep, grazing in six different locations in Western Australia, were reported to partially or completely refuse to consume annual Medicago pods, which were later found by Barbetti and Allen (2005) to be contaminated with a number of different Fusarium species. Subsequent rat toxigenicity studies by Barbetti and Allen (2006) on 16 of these Western Australian Fusarium species showed they can cause high mortality rates. Fusarium species from other parts of the world are known to produce deoxynivalenol (causes vomiting and feed refusal in animals) and/or diacetoxyscirpenol (also causes reduced feed intake in animals) as part of their array of toxigenic secondary metabolites. The aim of this study was to isolate and characterize the secondary metabolites produced by the 16 Fusarium species associated with sheep feed refusal disorders in Western Australia. Purification of toxigenic fungal extracts using a brine shrimp (Artemia salina) bioassay guided fractionation and HPLC, and identification by GC/MS and NMR spectroscopy, revealed the presence of a number of trichothecenes (3-acetyldeoxynivalenol, deoxynivalenol, nivalenol, monoacetoxyscirpenol, diacetoxyscirpenol, and scirpenol), enniatins (A1, B, and B1) and zearalenone. -

Wild Flowers of Western Australia
Wild Flowers of Western Australia Naturetrek Tour Report 31 August - 16 September 2007 Caladenia flava Caladenia hirta subsp. rosea Caladenia macrostylis Paracaleana terminalis Report and photos compiled by Paul Harmes Naturetrek Cheriton Mill Cheriton Alresford Hampshire SO24 0NG England T: +44 (0)1962 733051 F: +44 (0)1962 736426 E: [email protected] W: www.naturetrek.co.uk Tour Report Wild Flowers of Western Australia Tour Leaders: Paul Harmes Botanist Alan Notley Botanist Dave “Red” Morrell Driver Participants: Jane and David Crane Rita Hemsley Priscilla Nobbs Valerie Syrett Joan and David Vickers Dallas and Terry Wynne Day 1 Friday 31st August Weather: Warm and Sunny in London. Hot (35 degrees) in Dubai. Jane and David and Dallas and Terry met with Paul at the boarding gate, at Heathrow Terminal 3, for Emirates flight EK002 to Dubai, departing at 14-00hrs. Following a 7 hour flight we arrived in Dubai, and made our way to the boarding gate for the Emirates flight EK421 to Perth, where we met up with Rita and Priscilla, who had arrived via Gatwick. Day 2 Saturday 1st September Weather: Hot in Dubai. Fine warm and dry in Perth. The Emirates EK420 flight to Perth departed Dubai at 03-15hrs, arriving in Perth at 17-15hrs local time. After completing the immigration, customs and quarantine formalities, we met up with Red, our Australian driver for the duration of the tour. Red transported us into the city, and Miss Maud’s Swedish Hotel, our base for the next two nights. After settling into our rooms, we met up, in reception, with Valerie, Joan and Dave, as well as Alan Notley and his wife, Jahannah, and we all made our way into the restaurant for dinner. -
Australia's Floral Emblems
Floral Emblems Blossom on March 24 Stamps Two recent issues from Australia provide us with colorful flow- Australia’s state floral emblems also featured in the new ers and a salute to the 88th Birthday of Queen Elizabeth II. stamp issue are: Released on March 24, Australia Post featured national and • Tasmanian Blue Gum was proclaimed the floral emblem of state floral emblems that are striking examples of Australia’s flora Tasmania in 1962. and form part of the nation’s identity and floral heritage. • Waratah was proclaimed the floral emblem of New South Left to right, the four domestic base-rate (70¢) stamps depict Wales in 1962. the Golden Wattle Acacia pycnantha, Tasmanian Blue Gum Euca- • Common Heath was proclaimed the floral emblem of Vic- lyptus globulus, Waratah Telopea speciosissima and the Common toria in 1958. Heath Epacris impressa. • Cooktown Orchid was proclaimed the floral emblem of The three large letter rate ($1.40, $2.10 and $3.50) stamps de- Queensland in 1959. pict the Cooktown Orchid Dendrobium phalaenopsis, the Red and • Red and Green Kangaroo Paw was proclaimed the floral Green Kangaroo Paw Anigozanthos manglesii and Sturt’s Desert emblem of Western Australia in 1960. Pea Swainsona formosa respectively. • Sturt’s Desert Pea was proclaimed the floral emblem of “Australians have a love of flowers and these floral emblems South Australia in 1961. are not only visually stunning, but are symbolic of our nation,” said The associated products with this stamp issue include a first Australia Post Philatelic Manager, Mr Michael Zsolt. day cover, stamp pack, a maxicard set, two postcards, a medallion Australia’s national flower, the Golden Wattle is featured on cover, booklets of 10 and 20 x 70¢ self-adhesive stamps, and rolls one of the 70 cent stamps. -
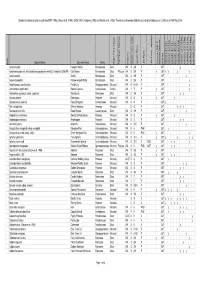
Bush Forever Species List
Species lists based on plot records from DEP (1996), Gibson et al. (1994), Griffin (1993), Keighery (1996) and Weston et al. (1992). Taxonomy and species attributes according to Keighery et al. (2006) as of 16th May 2005. Species Name Common Name Family Wd? Major Plant Group Significant Species Endemic Growth Form Code Growth Form Life Form Life Form - aquatics Common SSCP Wetland Species BFS No BRIX01 (FCT8) BRIX02 (FCT3a) BRIX03 (FCT8) BRIX04 (FCT8) BRIX05 (FCT3a) YULE01 (FCT23a) YULE02 (FCT23a) YULE03 (FCT21c) YULE04 (FCT10a) YULE05 (FCT7) Acacia huegelii Huegel's Wattle Mimosaceae Dicot WA 3 SH P 387 y Acacia lasiocarpa var. bracteolata long peduncle variant(G.J.Keighery 5026) PN Clay Moses Mimosaceae Dicot P1/p,s,e WA 3 SH P y 387 y y y y Acacia sessilis Wattle Mimosaceae Dicot WA 3 SH P 387 y Acacia stenoptera Narrow-winged Wattle Mimosaceae Dicot WA 3 SH P 387 y Acanthocarpus canaliculatus Prickle Lily Dasypogonaceae Monocot WA 4 H-SH P 387 y Actinostrobus pyramidalis Swamp Cypress Cupressaceae Conifer WA 1 T P y 387 y Adenanthos cygnorum subsp. cygnorum Woollybush Proteaceae Dicot WA 3 SH P 387 y y Agrostis plebeia Blowngrass Poaceae Monocot WA 5 G A y 387 y Agrostocrinum scabrum False Blindgrass Anthericaceae Monocot WA 4 H P 387 y * Aira caryophyllea Silvery Hairgrass Poaceae Monocot 5 G A 387 y y y Allocasuarina humilis Dwarf Sheoak Casuarinaceae Dicot WA 3 SH P 387 y Amphibromus nervosus Swamp Wallaby Grass Poaceae Monocot WA 5 G P y 387 y Amphipogon turbinatus Amphipogon Poaceae Monocot WA 5 G P 387 y y Anarthria gracilis Anarthria Restionaceae Monocot WA 6 S-R P 387 y Anigozanthos manglesii subsp. -
Brochure of Native Garden Species Resistant to Dieback
Native Garden Plants RESISTANT TO DIEBACK (Phytophthora cinnamomi) E. Groves, P. Hollick, G. Hardy and J. McComb Murdoch University 2009 The disease known as dieback disease or jarrah dieback is caused by the soilborne pathogen Phytophthora cinnamomi. This was introduced into Australia and has long been recognised as a serious threat to the flora in the jarrah forest and on the northern and southern sandplains. This list consists of Australian native plant species that are resistant to dieback and are available in WA nurseries. It has been compiled from field observations of resistance and the results of controlled experiments. However the classification of a plant as resistant to P. cinnamomi often depends on other environmental factors which can influence susceptibility to the pathogen. This list therefore provides a guide to plants which would have the greatest chance of survival in dieback affected sites. This list is organised under different plant types and superscripts indicate 1 - glasshouse inoculation experiment; 2 - field inoculation; 3 - field observations. Trees Name and Description Natural Distribution Acacia dealbata 3 Silver Wattle Medium tree, grey fern-like foliage and bright yellow flowers in spring NSW, VIC, TAS Acacia decurrens 3 Black Wattle Medium tree, bright green, fern-like foliage and profuse yellow flowers in early spring NSW Acacia fimbriata 3 Fringed Wattle Small tree, small leaves and masses of yellow flowers in spring QLD, NSW Acacia floribunda 3 Gossamer Wattle Tall shrub or small tree, slightly weeping, -

Molecular Systematics and Ecology of Invasive Kangaroo Paws in South Africa: Management Implications for a Horticulturally Important Genus
Biol Invasions (2010) 12:3989–4002 DOI 10.1007/s10530-010-9818-4 ORIGINAL PAPER Molecular systematics and ecology of invasive Kangaroo Paws in South Africa: management implications for a horticulturally important genus J. J. Le Roux • S. Geerts • P. Ivey • S. Krauss • D. M. Richardson • J. Suda • J. R. U. Wilson Received: 27 November 2009 / Accepted: 31 March 2010 / Published online: 2 July 2010 Ó Springer Science+Business Media B.V. 2010 Abstract Most legislation pertaining to non-native without bird pollination. Given the known propensity organisms is implicitly focussed at the individual of Kangaroo Paws to hybridise in their native range species level. However, in some cases interspecific in Australia, and confusion about the species identity hybrids can be more invasive than any of the parent of naturalized populations in South Africa, it was species. This is problematic for policy makers, and essential to resolve some key taxonomic issues in the for horticulturists developing or trading in new group. We constructed the first molecular phylogeny ornamental cultivars. We explore these issues in the for all species of the Kangaroo Paw group (genera context of the need to manage naturalized popula- Anigozanthos and Macropidia; family Haemodora- tions of Kangaroo Paws (Anigozanthos species) in ceae). As previously determined by taxonomists South Africa. Self-sustaining, dense populations of working on herbarium specimens, naturalized popu- naturalized Kangaroo Paws occur at several localities lations were identified as A. flavidus. In addition, we and are highly attractive to local nectar-feeding birds. also identified a second species, A. rufus. Relative The populations show high levels of seed set with or genome size estimates for Anigozanthos species J.