Combinatorial Readout of Unmodified H3R2 and Acetylated H3K14 by the Tandem PHD Finger of MOZ Reveals a Regulatory Mechanism for HOXA9 Transcription
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Expression Pattern and Level of ING5 Protein in Normal and Cancer Tissues
ONCOLOGY LETTERS 17: 63-68, 2019 Expression pattern and level of ING5 protein in normal and cancer tissues XUE-FENG YANG1, DAO-FU SHEN1, SHUANG ZHAO1, TIAN-REN REN2, YANG GAO1, SHUAI SHI1, JI-CHENG WU1, HONG-ZHI SUN1 and HUA-CHUAN ZHENG1,3 1Cancer Center and Key Laboratory of Brain and Spinal Cord Injury of Liaoning Province, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning 121001;2 Jilin Province Forestry Bureau, Linjiang, Jilin 134600; 3Institute of Life Sciences, Jinzhou Medical University, Jinzhou, Liaoning 121001, P.R. China Received February 21, 2016; Accepted February 13, 2017 DOI: 10.3892/ol.2018.9581 Abstract. Inhibitor of growth family 5 (ING5) functions as may be involved in cell regeneration and be associated with a type-II tumor suppressor gene and exerts an important role colorectal carcinogenesis. in DNA repair, apoptotic induction, proliferative inhibition, chromatin remodeling and the invasion process. In the present Introduction study, immunohistochemistry was performed to characterize the expression profile of ING5 protein on a tissue microarray Inhibitor of growth family 5 (ING5) is a member of the ING containing mouse and human normal tissues, and human protein family and functions as a type-II tumor suppressor cancer tissues, including hepatocellular (n=62), renal clear cell gene. Human ING5 is mapped to chromosome 2q37.3 and (n=62), pancreatic (n=62), esophageal squamous cell (n=45), encodes a 28-kDa protein with 240 amino acids (1). As indi- cervical squamous cell (n=31), breast (n=144), gastric (n=196), cated in Fig. 1, ING5 protein consists of a number of domains, colorectal (n=96), endometrial (n=96) and lung carcinoma including leucine zipper like (LZL), novel conserved region (n=192). -

The Tumor Suppressor ING5 Is a Dimeric, Bivalent Recognition Molecule of the Histone H3k4me3 Mark
Article The Tumor Suppressor ING5 Is a Dimeric, Bivalent Recognition Molecule of the Histone H3K4me3 Mark Georgina Ormaza 1,†, Jhon A. Rodríguez 1,†, Alain Ibáñez de Opakua 1, Nekane Merino 1, Maider Villate 1, Irantzu Gorroño 1, Miriam Rábano 1, Ignacio Palmero 2, Marta Vilaseca 3, Robert Kypta 1,4, María d.M. Vivanco 1, Adriana L. Rojas 1 and Francisco J. Blanco 1,5 1 - CIC bioGUNE, Parque Tecnológico de Bizkaia, 48160 Derio, Spain 2 - Instituto de Investigaciones Biomédicas “Alberto Sols”, CSIC-UAM, 28029 Madrid, Spain 3 - Institute for Research in Biomedicine, 08028 Barcelona, Spain 4 - Department of Surgery and Cancer, Imperial College London, London, W12 0NN, UK 5 - IKERBASQUE, Basque Foundation for Science, 48011 Bilbao, Spain Correspondence to Francisco J. Blanco: CIC bioGUNE, Parque Tecnológico de Bizkaia, 48160 Derio, Spain. [email protected] https://doi.org/10.1016/j.jmb.2019.04.018 Edited by M. Guss Abstract The INhibitor of Growth (ING) family of tumor suppressors regulates the transcriptional state of chromatin by recruiting remodeling complexes to sites with histone H3 trimethylated at lysine 4 (H3K4me3). This modification is recognized by the plant homeodomain (PHD) present at the C-terminus of the five ING proteins. ING5 facilitates histone H3 acetylation by the HBO1 complex, and also H4 acetylation by the MOZ/ MORF complex. We show that ING5 forms homodimers through its N-terminal domain, which folds independently into an elongated coiled-coil structure. The central region of ING5, which contains the nuclear localization sequence, is flexible and disordered, but it binds dsDNA with micromolar affinity. NMR analysis of the full-length protein reveals that the two PHD fingers of the dimer are chemically equivalent and independent of the rest of the molecule, and they bind H3K4me3 in the same way as the isolated PHD. -

Modes of Interaction of KMT2 Histone H3 Lysine 4 Methyltransferase/COMPASS Complexes with Chromatin
cells Review Modes of Interaction of KMT2 Histone H3 Lysine 4 Methyltransferase/COMPASS Complexes with Chromatin Agnieszka Bochy ´nska,Juliane Lüscher-Firzlaff and Bernhard Lüscher * ID Institute of Biochemistry and Molecular Biology, Medical School, RWTH Aachen University, Pauwelsstrasse 30, 52057 Aachen, Germany; [email protected] (A.B.); jluescher-fi[email protected] (J.L.-F.) * Correspondence: [email protected]; Tel.: +49-241-8088850; Fax: +49-241-8082427 Received: 18 January 2018; Accepted: 27 February 2018; Published: 2 March 2018 Abstract: Regulation of gene expression is achieved by sequence-specific transcriptional regulators, which convey the information that is contained in the sequence of DNA into RNA polymerase activity. This is achieved by the recruitment of transcriptional co-factors. One of the consequences of co-factor recruitment is the control of specific properties of nucleosomes, the basic units of chromatin, and their protein components, the core histones. The main principles are to regulate the position and the characteristics of nucleosomes. The latter includes modulating the composition of core histones and their variants that are integrated into nucleosomes, and the post-translational modification of these histones referred to as histone marks. One of these marks is the methylation of lysine 4 of the core histone H3 (H3K4). While mono-methylation of H3K4 (H3K4me1) is located preferentially at active enhancers, tri-methylation (H3K4me3) is a mark found at open and potentially active promoters. Thus, H3K4 methylation is typically associated with gene transcription. The class 2 lysine methyltransferases (KMTs) are the main enzymes that methylate H3K4. KMT2 enzymes function in complexes that contain a necessary core complex composed of WDR5, RBBP5, ASH2L, and DPY30, the so-called WRAD complex. -

Histone H3K4 Demethylation Is Negatively Regulated by Histone H3 Acetylation in Saccharomyces Cerevisiae
Histone H3K4 demethylation is negatively regulated by histone H3 acetylation in Saccharomyces cerevisiae Vicki E. Maltbya, Benjamin J. E. Martina, Julie Brind’Amourb,1, Adam T. Chruscickia,1, Kristina L. McBurneya, Julia M. Schulzec, Ian J. Johnsona, Mark Hillsd, Thomas Hentrichc, Michael S. Koborc, Matthew C. Lorinczb, and LeAnn J. Howea,2 Departments of aBiochemistry and Molecular Biology and bMedical Genetics, Life Sciences Institute, University of British Columbia, Vancouver, BC, Canada V6T 1Z3; cCenter for Molecular Medicine and Therapeutics, Child and Family Research Institute, Vancouver, BC, Canada V5Z 4H4; and dTerry Fox Laboratory, British Columbia Cancer Agency, Vancouver, BC, Canada V5Z 1L3 Edited by Kevin Struhl, Harvard Medical School, Boston, MA, and approved September 12, 2012 (received for review February 6, 2012) Histone H3 lysine 4 trimethylation (H3K4me3) is a hallmark of of lysine-specific HDMs have been identified: amine oxidases, transcription initiation, but how H3K4me3 is demethylated during such as LSD1, and the JmjC (Jumonji C) domain–containing gene repression is poorly understood. Jhd2, a JmjC domain protein, demethylases. This latter class of demethylases can be split fur- was recently identified as the major H3K4me3 histone demethylase ther into several subfamilies, including the evolutionarily con- (HDM) in Saccharomyces cerevisiae. Although JHD2 is required for served JARID1 family of demethylases, which is characterized JHD2 removal of methylation upon gene repression, deletion of does not only by a JmjC domain but also by JmjN, AT-rich interactive, not result in increased levels of H3K4me3 in bulk histones, indicating C5HC2 zinc finger, and PHD finger domains (6). In the yeast S. that this HDM is unable to demethylate histones during steady-state cerevisiae, the lone member of the JARID1 family of demethy- conditions. -

The Role of ING5 in Maintaining Stemness of Brain Tumor Initiating Cells
University of Calgary PRISM: University of Calgary's Digital Repository Graduate Studies The Vault: Electronic Theses and Dissertations 2016 The Role of ING5 in Maintaining Stemness of Brain Tumor Initiating Cells Wang, Fangwu Jr Wang, F. J. (2016). The Role of ING5 in Maintaining Stemness of Brain Tumor Initiating Cells (Unpublished master's thesis). University of Calgary, Calgary, AB. doi:10.11575/PRISM/28327 http://hdl.handle.net/11023/3233 master thesis University of Calgary graduate students retain copyright ownership and moral rights for their thesis. You may use this material in any way that is permitted by the Copyright Act or through licensing that has been assigned to the document. For uses that are not allowable under copyright legislation or licensing, you are required to seek permission. Downloaded from PRISM: https://prism.ucalgary.ca UNIVERSITY OF CALGARY The Role of ING5 in Maintaining Stemness of Brain Tumor Initiating Cells by Fangwu Wang A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE GRADUATE PROGRAM IN BIOCHEMISTRY AND MOLECULAR BIOLOGY CALGARY, ALBERTA AUGUST, 2016 © Fangwu Wang 2016 Abstract Brain tumor initiating cells (BTICs) are believed to account for the recurrence of glioblastomas following treatment. Recent studies have shown that the stemness of BTICs and intratumoral differentiation hierarchy are determined largely on the epigenetic level. The ING family of epigenetic regulators function in diverse growth regulatory, metastasis and chemoresistance pathways, through targeting different histone acetyltransferase (HAT) and histone deacetylase (HDAC) complexes to the H3K4me3 mark to alter histone acetylation. -

Recognition of Histone Acetylation by the GAS41 YEATS Domain Promotes H2A.Z Deposition in Non-Small Cell Lung Cancer
Downloaded from genesdev.cshlp.org on October 5, 2021 - Published by Cold Spring Harbor Laboratory Press Recognition of histone acetylation by the GAS41 YEATS domain promotes H2A.Z deposition in non-small cell lung cancer Chih-Chao Hsu,1,2,8 Jiejun Shi,3,8 Chao Yuan,1,2,7,8 Dan Zhao,4,5,8 Shiming Jiang,1,2 Jie Lyu,3 Xiaolu Wang,1,2 Haitao Li,4,5 Hong Wen,1,2 Wei Li,3 and Xiaobing Shi1,2,6 1Department of Epigenetics and Molecular Carcinogenesis, The University of Texas MD Anderson Cancer Center, Houston, Texas 77030, USA; 2Center for Cancer Epigenetics, The University of Texas MD Anderson Cancer Center, Houston, Texas 77030, USA; 3Dan L. Duncan Cancer Center, Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, Texas 77030, USA; 4MOE Key Laboratory of Protein Sciences, Beijing Advanced Innovation Center for Structural Biology, Department of Basic Medical Sciences, School of Medicine, Tsinghua University, Beijing 100084, China; 5Tsinghua-Peking Joint Center for Life Sciences, Tsinghua University, Beijing 100084, China; 6Genetics and Epigenetics Graduate Program, The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences, Houston, Texas 77030, USA Histone acetylation is associated with active transcription in eukaryotic cells. It helps to open up the chromatin by neutralizing the positive charge of histone lysine residues and providing binding platforms for “reader” proteins. The bromodomain (BRD) has long been thought to be the sole protein module that recognizes acetylated histones. Re- cently, we identified the YEATS domain of AF9 (ALL1 fused gene from chromosome 9) as a novel acetyl-lysine- binding module and showed that the ENL (eleven-nineteen leukemia) YEATS domain is an essential acetyl-histone reader in acute myeloid leukemias. -

Deciphering the Histone Code to Build the Genome Structure
bioRxiv preprint doi: https://doi.org/10.1101/217190; this version posted November 20, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Deciphering the histone code to build the genome structure Kirti Prakasha,b,c,* and David Fournierd,* aPhysico-Chimie Curie, Institut Curie, CNRS UMR 168, 75005 Paris, France; bOxford Nanoimaging Ltd, OX1 1JD, Oxford, UK; cMicron Advanced Bioimaging Unit, Department of Biochemistry, University of Oxford, Oxford, UK; dFaculty of Biology and Center for Computational Sciences, Johannes Gutenberg University Mainz, 55128 Mainz, Germany; *Correspondence: [email protected], [email protected] Histones are punctuated with small chemical modifications that alter their interaction with DNA. One attractive hypothesis stipulates that certain combinations of these histone modifications may function, alone or together, as a part of a predictive histone code to provide ground rules for chromatin folding. We consider four features that relate histone modifications to chromatin folding: charge neutrali- sation, molecular specificity, robustness and evolvability. Next, we present evidence for the association among different histone modi- fications at various levels of chromatin organisation and show how these relationships relate to function such as transcription, replica- tion and cell division. Finally, we propose a model where the histone code can set critical checkpoints for chromatin to fold reversibly be- tween different orders of the organisation in response to a biological stimulus. DNA | nucleosomes | histone modifications | chromatin domains | chro- mosomes | histone code | chromatin folding | genome structure Introduction The genetic information within chromosomes of eukaryotes is packaged into chromatin, a long and folded polymer of double-stranded DNA, histones and other structural and non- structural proteins. -

Open Data for Differential Network Analysis in Glioma
International Journal of Molecular Sciences Article Open Data for Differential Network Analysis in Glioma , Claire Jean-Quartier * y , Fleur Jeanquartier y and Andreas Holzinger Holzinger Group HCI-KDD, Institute for Medical Informatics, Statistics and Documentation, Medical University Graz, Auenbruggerplatz 2/V, 8036 Graz, Austria; [email protected] (F.J.); [email protected] (A.H.) * Correspondence: [email protected] These authors contributed equally to this work. y Received: 27 October 2019; Accepted: 3 January 2020; Published: 15 January 2020 Abstract: The complexity of cancer diseases demands bioinformatic techniques and translational research based on big data and personalized medicine. Open data enables researchers to accelerate cancer studies, save resources and foster collaboration. Several tools and programming approaches are available for analyzing data, including annotation, clustering, comparison and extrapolation, merging, enrichment, functional association and statistics. We exploit openly available data via cancer gene expression analysis, we apply refinement as well as enrichment analysis via gene ontology and conclude with graph-based visualization of involved protein interaction networks as a basis for signaling. The different databases allowed for the construction of huge networks or specified ones consisting of high-confidence interactions only. Several genes associated to glioma were isolated via a network analysis from top hub nodes as well as from an outlier analysis. The latter approach highlights a mitogen-activated protein kinase next to a member of histondeacetylases and a protein phosphatase as genes uncommonly associated with glioma. Cluster analysis from top hub nodes lists several identified glioma-associated gene products to function within protein complexes, including epidermal growth factors as well as cell cycle proteins or RAS proto-oncogenes. -

Transcription Shapes Genome-Wide Histone Acetylation Patterns
ARTICLE https://doi.org/10.1038/s41467-020-20543-z OPEN Transcription shapes genome-wide histone acetylation patterns Benjamin J. E. Martin 1, Julie Brind’Amour 2, Anastasia Kuzmin1, Kristoffer N. Jensen2, Zhen Cheng Liu1, ✉ Matthew Lorincz 2 & LeAnn J. Howe 1 Histone acetylation is a ubiquitous hallmark of transcription, but whether the link between histone acetylation and transcription is causal or consequential has not been addressed. 1234567890():,; Using immunoblot and chromatin immunoprecipitation-sequencing in S. cerevisiae, here we show that the majority of histone acetylation is dependent on transcription. This dependency is partially explained by the requirement of RNA polymerase II (RNAPII) for the interaction of H4 histone acetyltransferases (HATs) with gene bodies. Our data also confirms the targeting of HATs by transcription activators, but interestingly, promoter-bound HATs are unable to acetylate histones in the absence of transcription. Indeed, HAT occupancy alone poorly predicts histone acetylation genome-wide, suggesting that HAT activity is regulated post- recruitment. Consistent with this, we show that histone acetylation increases at nucleosomes predicted to stall RNAPII, supporting the hypothesis that this modification is dependent on nucleosome disruption during transcription. Collectively, these data show that histone acetylation is a consequence of RNAPII promoting both the recruitment and activity of histone acetyltransferases. 1 Department of Biochemistry and Molecular Biology, Life Sciences Institute, Molecular -

Histone Modifications During the Life Cycle of the Brown Alga Ectocarpus
Bourdareau et al. Genome Biology (2021) 22:12 https://doi.org/10.1186/s13059-020-02216-8 RESEARCH Open Access Histone modifications during the life cycle of the brown alga Ectocarpus Simon Bourdareau1, Leila Tirichine2, Bérangère Lombard3, Damarys Loew3, Delphine Scornet1, Yue Wu2, Susana M. Coelho1,4* and J. Mark Cock1* * Correspondence: susana.coelho@ tuebingen.mpg.de; cock@sb-roscoff. Abstract fr 1CNRS, Sorbonne Université, UPMC Background: Brown algae evolved complex multicellularity independently of the University Paris 06, Algal Genetics animal and land plant lineages and are the third most developmentally complex Group, UMR 8227, Integrative phylogenetic group on the planet. An understanding of developmental processes in Biology of Marine Models, Station Biologique de Roscoff, CS 90074, this group is expected to provide important insights into the evolutionary events F-29688 Roscoff, France necessary for the emergence of complex multicellularity. Here, we focus on Full list of author information is mechanisms of epigenetic regulation involving post-translational modifications of available at the end of the article histone proteins. Results: A total of 47 histone post-translational modifications are identified, including a novel mark H2AZR38me1, but Ectocarpus lacks both H3K27me3 and the major polycomb complexes. ChIP-seq identifies modifications associated with transcription start sites and gene bodies of active genes and with transposons. H3K79me2 exhibits an unusual pattern, often marking large genomic regions spanning several genes. Transcription start sites of closely spaced, divergently transcribed gene pairs share a common nucleosome-depleted region and exhibit shared histone modification peaks. Overall, patterns of histone modifications are stable through the life cycle. Analysis of histone modifications at generation-biased genes identifies a correlation between the presence of specific chromatin marks and the level of gene expression. -

Histone Methylation Regulation in Neurodegenerative Disorders
International Journal of Molecular Sciences Review Histone Methylation Regulation in Neurodegenerative Disorders Balapal S. Basavarajappa 1,2,3,4,* and Shivakumar Subbanna 1 1 Division of Analytical Psychopharmacology, Nathan Kline Institute for Psychiatric Research, Orangeburg, NY 10962, USA; [email protected] 2 New York State Psychiatric Institute, New York, NY 10032, USA 3 Department of Psychiatry, College of Physicians & Surgeons, Columbia University, New York, NY 10032, USA 4 New York University Langone Medical Center, Department of Psychiatry, New York, NY 10016, USA * Correspondence: [email protected]; Tel.: +1-845-398-3234; Fax: +1-845-398-5451 Abstract: Advances achieved with molecular biology and genomics technologies have permitted investigators to discover epigenetic mechanisms, such as DNA methylation and histone posttransla- tional modifications, which are critical for gene expression in almost all tissues and in brain health and disease. These advances have influenced much interest in understanding the dysregulation of epigenetic mechanisms in neurodegenerative disorders. Although these disorders diverge in their fundamental causes and pathophysiology, several involve the dysregulation of histone methylation- mediated gene expression. Interestingly, epigenetic remodeling via histone methylation in specific brain regions has been suggested to play a critical function in the neurobiology of psychiatric disor- ders, including that related to neurodegenerative diseases. Prominently, epigenetic dysregulation currently brings considerable interest as an essential player in neurodegenerative disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), Amyotrophic lateral sclerosis (ALS) and drugs of abuse, including alcohol abuse disorder, where it may facilitate connections between genetic and environmental risk factors or directly influence disease-specific Citation: Basavarajappa, B.S.; Subbanna, S. -
Download Validation Data
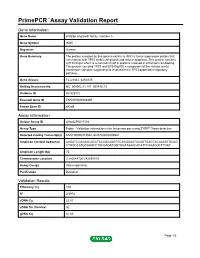
PrimePCR™Assay Validation Report Gene Information Gene Name inhibitor of growth family, member 5 Gene Symbol ING5 Organism Human Gene Summary The protein encoded by this gene is similar to ING1 a tumor suppressor protein that can interact with TP53 inhibit cell growth and induce apoptosis. This protein contains a PHD-finger which is a common motif in proteins involved in chromatin remodeling. This protein can bind TP53 and EP300/p300 a component of the histone acetyl transferase complex suggesting its involvement in TP53-dependent regulatory pathway. Gene Aliases FLJ23842, p28ING5 RefSeq Accession No. NC_000002.11, NT_005416.13 UniGene ID Hs.529172 Ensembl Gene ID ENSG00000168395 Entrez Gene ID 84289 Assay Information Unique Assay ID qHsaCIP0031594 Assay Type Probe - Validation information is for the primer pair using SYBR® Green detection Detected Coding Transcript(s) ENST00000313552, ENST00000406941 Amplicon Context Sequence AAGATCCAGAACGCCTACAGCAAGTGCAAGGAATACAGTGACGACAAAGTGCAG CTGGCCATGCAGACCTACGAGATGGTGGATAAACACATTCGAAGGCTTGAT Amplicon Length (bp) 75 Chromosome Location 2:242648720-242650818 Assay Design Intron-spanning Purification Desalted Validation Results Efficiency (%) 100 R2 0.9978 cDNA Cq 22.07 cDNA Tm (Celsius) 82 gDNA Cq 41.86 Page 1/5 PrimePCR™Assay Validation Report Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 2/5 PrimePCR™Assay Validation Report ING5, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak