Geochemistry of Persististrombus Latus Gmelin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

JVP 26(3) September 2006—ABSTRACTS
Neoceti Symposium, Saturday 8:45 acid-prepared osteolepiforms Medoevia and Gogonasus has offered strong support for BODY SIZE AND CRYPTIC TROPHIC SEPARATION OF GENERALIZED Jarvik’s interpretation, but Eusthenopteron itself has not been reexamined in detail. PIERCE-FEEDING CETACEANS: THE ROLE OF FEEDING DIVERSITY DUR- Uncertainty has persisted about the relationship between the large endoskeletal “fenestra ING THE RISE OF THE NEOCETI endochoanalis” and the apparently much smaller choana, and about the occlusion of upper ADAM, Peter, Univ. of California, Los Angeles, Los Angeles, CA; JETT, Kristin, Univ. of and lower jaw fangs relative to the choana. California, Davis, Davis, CA; OLSON, Joshua, Univ. of California, Los Angeles, Los A CT scan investigation of a large skull of Eusthenopteron, carried out in collaboration Angeles, CA with University of Texas and Parc de Miguasha, offers an opportunity to image and digital- Marine mammals with homodont dentition and relatively little specialization of the feeding ly “dissect” a complete three-dimensional snout region. We find that a choana is indeed apparatus are often categorized as generalist eaters of squid and fish. However, analyses of present, somewhat narrower but otherwise similar to that described by Jarvik. It does not many modern ecosystems reveal the importance of body size in determining trophic parti- receive the anterior coronoid fang, which bites mesial to the edge of the dermopalatine and tioning and diversity among predators. We established relationships between body sizes of is received by a pit in that bone. The fenestra endochoanalis is partly floored by the vomer extant cetaceans and their prey in order to infer prey size and potential trophic separation of and the dermopalatine, restricting the choana to the lateral part of the fenestra. -

Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/319999645 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Chapter · September 2017 DOI: 10.1002/9781119154051.ch10 CITATIONS READS 0 332 28 authors, including: Nelson A Lagos Ricardo Norambuena University Santo Tomás (Chile) University of Concepción 65 PUBLICATIONS 1,052 CITATIONS 13 PUBLICATIONS 252 CITATIONS SEE PROFILE SEE PROFILE Claudio Silva Marco A Lardies Pontificia Universidad Católica de Valparaíso Universidad Adolfo Ibáñez 54 PUBLICATIONS 432 CITATIONS 70 PUBLICATIONS 1,581 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Irish moss - green crab interactions View project Influence of environment on fish stock assessment View project All content following this page was uploaded by Pedro A. Quijón on 11 November 2017. The user has requested enhancement of the downloaded file. 239 10 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Eleuterio Yáñez1, Nelson A. Lagos2,13, Ricardo Norambuena3, Claudio Silva1, Jaime Letelier4, Karl-Peter Muck5, Gustavo San Martin6, Samanta Benítez2,13, Bernardo R. Broitman7,13, Heraldo Contreras8, Cristian Duarte9,13, Stefan Gelcich10,13, Fabio A. Labra2, Marco A. Lardies11,13, Patricio H. Manríquez7, Pedro A. Quijón12, Laura Ramajo2,11, Exequiel González1, Renato Molina14, Allan Gómez1, Luis Soto15, Aldo Montecino16, María Ángela Barbieri17, Francisco Plaza18, Felipe Sánchez18, -

Growth of Florida Fighting Conch, Strombus Alatus, in Recirculating Systems
Contribution No. 666 Growth of Florida Fighting Conch, Strombus alatus, in Recirculating Systems 2 AMBER SHAWL', DAVE JENKINS , :tvlEGAN DAVIS I, and KEVAN MAIN2 I Harbor Branch Oceanographic Institution Aquaculture Division 5600 US 1 North Ft. Pierce, Florida 34946 USA [email protected],· mdavis@hboi. edu 2 Mote Marine Laboratory 1600 Ken Thompson Parkway Sarasota, Florida 34236 USA [email protected],· [email protected] ABSTRACT With the increased interest in water conservation and the need to reduce the discharge of effluent from aquaculture production systems, there has been a shift from open, flow-through systems to recirculating aquaculture production systems. In 2001, Harbor Branch Oceanographic Institution developed the first recirculating conch aquaculture program. Two important aspects of conch aquaculture are detennining the stocking density and water quality parameters in growout systems that yield the fastest growth rate and the highest survival. An experiment was conducted from March 11 - June 3, 2003 at two Florida sites, Harbor Branch Oceanographic Institution and Mote Marine Laboratory, to compare survival and growth of juvenile conch in a recirculating growout system. The recirculating system consisted of raceways troughs with an elevated sand substrate. The three replicate raceway troughs at each site were stocked with juvenile (16.9 ± 1.9 shell length) Florida fighting conch, Strom bus alatus at 75 conchlm2 (l09 and 140 conch per replicate at Harbor Branch and Mote, respectively). In 12 weeks, the conch grew 18.8 mm or 0.22 mmI day at Harbor Branch and 22.5 mm or 0.27 mmJday at Mote. There was a significantly faster growth rate at Mote, which appeared to be due to a lower stocking density throughout the experiment. -

First Report of Ancylostoma Ceylanicum in Wild Canids Q ⇑ Felicity A
International Journal for Parasitology: Parasites and Wildlife 2 (2013) 173–177 Contents lists available at SciVerse ScienceDirect International Journal for Parasitology: Parasites and Wildlife journal homepage: www.elsevier.com/locate/ijppaw Brief Report First report of Ancylostoma ceylanicum in wild canids q ⇑ Felicity A. Smout a, R.C. Andrew Thompson b, Lee F. Skerratt a, a School of Public Health, Tropical Medicine and Rehabilitation Sciences, James Cook University, Townsville, Queensland 4811, Australia b School of Veterinary and Biomedical Sciences, Murdoch University, Murdoch, Western Australia 6150, Australia article info abstract Article history: The parasitic nematode Ancylostoma ceylanicum is common in dogs, cats and humans throughout Asia, Received 19 February 2013 inhabiting the small intestine and possibly leading to iron-deficient anaemia in those infected. It has pre- Revised 23 April 2013 viously been discovered in domestic dogs in Australia and this is the first report of A. ceylanicum in wild Accepted 26 April 2013 canids. Wild dogs (dingoes and dingo hybrids) killed in council control operations (n = 26) and wild dog scats (n = 89) were collected from the Wet Tropics region around Cairns, Far North Queensland. All of the carcasses (100%) were infected with Ancylostoma caninum and three (11.5%) had dual infections with A. Keywords: ceylanicum. Scats, positively sequenced for hookworm, contained A. ceylanicum, A. caninum and Ancylos- Ancylostoma ceylanicum toma braziliense, with A. ceylanicum the dominant species in Mount Windsor National Park, with a prev- Dingo Canine alence of 100%, but decreasing to 68% and 30.8% in scats collected from northern and southern rural Hookworm suburbs of Cairns, respectively. -

References Please Help Making This Preliminary List As Complete As Possible!
Cypraeidae - important references Please help making this preliminary list as complete as possible! ABBOTT, R.T. (1965) Cypraea arenosa Gray, 1825. Hawaiian Shell News 14(2):8 ABREA, N.S. (1980) Strange goings on among the Cypraea ziczac. Hawaiian Shell News 28 (5):4 ADEGOKE, O.S. (1973) Paleocene mollusks from Ewekoro, southern Nigeria. Malacologia 14:19-27, figs. 1-2, pls. 1-2. ADEGOKE, O.S. (1977) Stratigraphy and paleontology of the Ewekoro Formation (Paleocene) of southeastern Nigeria. Bulletins of American Paleontology 71(295):1-379, figs. 1-6, pls. 1-50. AIKEN, R. P. (2016) Description of two undescribed subspecies and one fossil species of the Genus Cypraeovula Gray, 1824 from South Africa. Beautifulcowries Magazine 8: 14-22 AIKEN, R., JOOSTE, P. & ELS, M. (2010) Cypraeovula capensis - A specie of Diversity and Beauty. Strandloper 287 p. 16 ff AIKEN, R., JOOSTE, P. & ELS, M. (2014) Cypraeovula capensis. A species of diversity and beauty. Beautifulcowries Magazine 5: 38–44 ALLAN, J. (1956) Cowry Shells of World Seas. Georgian House, Melbourne, Australia, 170 p., pls. 1-15. AMANO, K. (1992) Cypraea ohiroi and its associated molluscan species from the Miocene Kadonosawa Formation, northeast Japan. Bulletin of the Mizunami Fossil Museum 19:405-411, figs. 1-2, pl. 57. ANCEY, C.F. (1901) Cypraea citrina Gray. The Nautilus 15(7):83. ANONOMOUS. (1971) Malacological news. La Conchiglia 13(146-147):19-20, 5 unnumbered figs. ANONYMOUS. (1925) Index and errata. The Zoological Journal. 1: [593]-[603] January. ANONYMOUS. (1889) Cypraea venusta Sowb. The Nautilus 3(5):60. ANONYMOUS. (1893) Remarks on a new species of Cypraea. -

Concholepas Concholepas
ANNALS OF THE SOUTH AFRICAN MUSEUM ANNALE VAN DIE SUID-AFRIKAANSE MUSEUM Volume 97 Band October 1985 Oktober Part 1 Deel THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPAS CONCHOLEPAS By BRIAN KENSLEY are issued in parts at irregular intervals as material becomes available word uitgegee in dele op ongereelde tye na gelang van die beskikbaarheid van stof 1,2(1-3,5-8),3(1-2,4-5,8, I.-p.i.), 5(1-3, 5, 7-9), 6(1, I.-p.i.), 7(1-4), 8, 9(1-2, 7), 10(1-3), 11(1-2,5,7, I.-p.i.), 14(1-2), 15(4-5),24(2),27,31(1-3),32(5),33,36(2),45(1) Printed in South Africa by In Suid-Afrika gedruk deur The Rustica Press, Pty., Ltd., Die Rustica-pers, Edms., Bpk., Court Road, Wynberg, Cape Courtweg, Wynberg, Kaap THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPASCONCHOLEPAS BRIAN KENSLEY National Museum of Natural History, Smithsonian Institution, Washington, D. C. The occurrence of the thaidid gastropod genus Concholepas is recorded from presumed Late Pleistocene coastal deposits in southern South West Africa-Namibia. The material is indistinguishable from C. concholepas, a species known from the Pliocene to Recent on the west coast of South America. The living species characteristically occurs in cold-temperate waters from the intertidal to depths of 40 m. It is suggested that the southern African fossils represent a short-lived pioneer population, established by larvae drifting from South America. -

Impact of the the COVID-19 Pandemic on a Queen Conch (Aliger Gigas) Fishery in the Bahamas
Impact of the the COVID-19 pandemic on a queen conch (Aliger gigas) fishery in The Bahamas Nicholas D. Higgs Cape Eleuthera Institute, Rock Sound, Eleuthera, Bahamas ABSTRACT The onset of the coronavirus (COVID-19) pandemic in early 2020 led to a dramatic rise in unemployment and fears about food-security throughout the Caribbean region. Subsistence fisheries were one of the few activities permitted during emergency lockdown in The Bahamas, leading many to turn to the sea for food. Detailed monitoring of a small-scale subsistence fishery for queen conch was undertaken during the implementation of coronavirus emergency control measures over a period of twelve weeks. Weekly landings data showed a surge in fishing during the first three weeks where landings were 3.4 times higher than subsequent weeks. Overall 90% of the catch was below the minimum legal-size threshold and individual yield declined by 22% during the lockdown period. This study highlights the role of small-scale fisheries as a `natural insurance' against socio-economic shocks and a source of resilience for small island communities at times of crisis. It also underscores the risks to food security and long- term sustainability of fishery stocks posed by overexploitation of natural resources. Subjects Aquaculture, Fisheries and Fish Science, Conservation Biology, Marine Biology, Coupled Natural and Human Systems, Natural Resource Management Keywords Fisheries, Coronavirus, COVID-19, IUU, SIDS, SDG14, Food security, Caribbean, Resiliance, Small-scale fisheries Submitted 2 December 2020 INTRODUCTION Accepted 16 July 2021 Published 3 August 2021 Subsistence fishing has played an integral role in sustaining island communities for Corresponding author thousands of years, especially small islands with limited terrestrial resources (Keegan et Nicholas D. -

Ensiklopedia Keanekaragaman Invertebrata Di Zona Intertidal Pantai Gesing Sebagai Sumber Belajar
ENSIKLOPEDIA KEANEKARAGAMAN INVERTEBRATA DI ZONA INTERTIDAL PANTAI GESING SEBAGAI SUMBER BELAJAR SKRIPSI Untuk memenuhi sebagian persyaratan mencapai derajat Sarjana S-1 Program Studi Pendidikan Biologi Diajukan Oleh Tika Dwi Yuniarti 15680036 PROGRAM STUDI PENDIDIKAN BIOLOGI FAKULTAS SAINS DAN TEKNOLOGI UIN SUNAN KALIJAGA YOGYAKARTA 2019 ii iii iv MOTTO إ َّن َم َع إلْ ُع ْ ِْس يُ ْ ًْسإ ٦ ِ Sesungguhnya sesudah kesulitan itu ada kemudahan (Q.S. Al-Insyirah: 6) “Hidup tanpa masalah berarti mati” (Lessing) v HALAMAN PERSEMBAHAN Skripsi ini saya persembahkan untuk: Keluargaku: Ibu, bapak, dan kakak tercinta Simbah Kakung dan Simbah Putri yang selalu saya cintai Keluarga di D.I. Yogyakarta Teman-teman seperjuangan Pendidikan Biologi 2015 Almamater tercinta: Program Studi Pendidikan Biologi Fakultas Sains dan Teknologi Universitas Islam Negeri Sunan Kalijaga Yogyakarta vi KATA PENGANTAR Puji syukur penulis panjatkan atas kehadirat Allah Yang Maha Pengasih lagi Maha Penyayang yang telah melimpahkan segala rahmat dan hidayah-Nya sehingga penulis dapat menyelesaikan skripsi ini dengan baik. Selawat serta salam senantiasa tercurah kepada Nabi Muhammad SAW beserta keluarga dan sahabatnya. Skripsi ini dapat diselesaikan berkat bimbingan, arahan, dan bantuan dari berbagai pihak. Oleh karena itu, penulis mengucapkan terimakasih kepada: 1. Bapak Dr. Murtono, M.Si., selaku Dekan Fakultas Sains dan Teknologi UIN Sunan Kalijaga Yogyakarta. 2. Bapak M. Ja’far Luthfi, Ph.D., selaku Wakil Dekan Fakultas Sains dan Teknologi. 3. Bapak Dr. Widodo, S.Pd., M.Pd., selaku Ketua Program Studi Pendidikan Biologi 4. Ibu Sulistyawati, S.Pd.I., M.Si., selaku dosen pembimbing skripsi dan dosen pembimbing akademik yang selalu mengarahkan dan memberikan banyak ilmu selama menjadi mahasiswa Pendidikan Biologi. -

AMERICAN MUSEUM NOVITATES Published by Number 1314 the AMERICAN MUSEUM of NATURAL HISTORY March 14, 1946 New York City
AMERICAN MUSEUM NOVITATES Published by Number 1314 THE AMERICAN MUSEUM OF NATURAL HISTORY March 14, 1946 New York City NOTES ON STROMBUS DENTATUS LINNE AND THE STROMBUS URCEUS COMPLEX BY HENRY DODGE A remark made by Tryon in his mono- latus Schumacher, 1817. This complex graph on the Strombidae (1885, p. 118) in presents such extremes of form, however, discussing Strombus dentatus Linn6 has that further study may justify its separa- prompted me to examine the taxonomic tion into subspecies or even species. history of the species in the so-called den- tatus group. Tryon there said: "The 1 difference between this species and S. On the first point we should turn im- urceus is so slight, and there is so much mediately to the Linnaean descriptions: variation in the shells, that it is very Strombus urceus LINNAEUS, 1758, p. 745, No. doubtful whether can be 440; 1767, p. 1212, No. 512. their separation "S. testae [sic] labro attenuato retuso brevi maintained." striato, ventre spiraque plicato-nodosis, apertura A reading of the meager references to bilabiata inermi." this group, a study of the synonyms in- TRANSLATION: Shells with a "thinned-out," of a reflected, short, and ridged lip, body-whorl and volved, and an examination consider- spire plicate-nodose, aperture bilabiate and lack- able series of specimens disclose an ob- ing armature. vious confusion which appeared as early as Strombus dentatu8 LINNAEUS, 1758, p. 745, Gmelin and has persisted in the minds of No. "o"; 1767, p. 1213, No. 513. I have "S. testa labro attenuato brevi dentato, ventre all but a few authors since his day. -

The Common Snapping Turtle, Chelydra Serpentina
The Common Snapping Turtle, Chelydra serpentina Rylen Nakama FISH 423: Olden 12/5/14 Figure 1. The Common Snapping Turtle, one of the most widespread reptiles in North America. Photo taken in Quebec, Canada. Image from https://www.flickr.com/photos/yorthopia/7626614760/. Classification Order: Testudines Family: Chelydridae Genus: Chelydra Species: serpentina (Linnaeus, 1758) Previous research on Chelydra serpentina (Phillips et al., 1996) acknowledged four subspecies, C. s. serpentina (Northern U.S. and Figure 2. Side profile of Chelydra serpentina. Note Canada), C. s. osceola (Southeastern U.S.), C. s. the serrated posterior end of the carapace and the rossignonii (Central America), and C. s. tail’s raised central ridge. Photo from http://pelotes.jea.com/AnimalFact/Reptile/snapturt.ht acutirostris (South America). Recent IUCN m. reclassification of chelonians based on genetic analyses (Rhodin et al., 2010) elevated C. s. rossignonii and C. s. acutirostris to species level and established C. s. osceola as a synonym for C. s. serpentina, thus eliminating subspecies within C. serpentina. Antiquated distinctions between the two formerly recognized North American subspecies were based on negligible morphometric variations between the two populations. Interbreeding in the overlapping range of the two populations was well documented, further discrediting the validity of the subspecies distinction (Feuer, 1971; Aresco and Gunzburger, 2007). Therefore, any emphasis of subspecies differentiation in the ensuing literature should be disregarded. Figure 3. Front-view of a captured Chelydra Continued usage of invalid subspecies names is serpentina. Different skin textures and the distinctive pink mouth are visible from this angle. Photo from still prevalent in the exotic pet trade for C. -
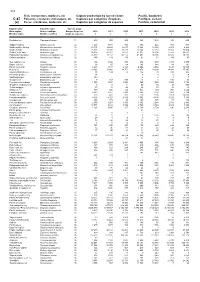
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
534 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southeast C-87 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2010 2011 2012 2013 2014 2015 2016 Nombre inglés Nombre científico Grupo de especies t t t t t t t Flatfishes nei Pleuronectiformes 31 613 302 806 323 1 804 483 693 Tadpole codling Salilota australis 32 1 400 1 091 768 374 522 703 536 Southern blue whiting Micromesistius australis 32 23 301 19 629 16 675 15 304 10 036 8 809 8 269 Southern hake Merluccius australis 32 25 361 20 909 20 288 19 346 12 393 16 150 16 804 South Pacific hake Merluccius gayi 32 90 305 82 977 72 872 92 031 96 196 77 283 98 662 Patagonian grenadier Macruronus magellanicus 32 74 330 70 137 62 175 47 602 39 138 37 475 28 108 Chilean grenadier Coelorinchus chilensis 32 156 134 136 91 54 59 47 Sea catfishes nei Ariidae 33 406 1 426 852 876 1 217 1 185 1 285 Snake eels nei Ophichthidae 33 38 65 114 142 144 49 181 Pacific cornetfish Fistularia corneta 33 6 443 4 513 4 323 4 854 2 534 7 630 19 559 Mullets nei Mugilidae 33 10 821 13 400 18 751 13 876 14 290 14 044 17 345 Snooks(=Robalos) nei Centropomus spp 33 98 104 78 136 79 310 305 Broomtail grouper Mycteroperca xenarcha 33 14 .. -

Size at Maturation, Spawning Variability and Fecundity in the Queen Conch, Aliger Gigas
Gulf and Caribbean Research Volume 31 Issue 1 2020 Size at Maturation, Spawning Variability and Fecundity in the Queen Conch, Aliger gigas Richard S. Appeldoorn GCFIMembers, [email protected] Follow this and additional works at: https://aquila.usm.edu/gcr Part of the Marine Biology Commons, and the Population Biology Commons To access the supplemental data associated with this article, CLICK HERE. Recommended Citation Appeldoorn, R. S. 2020. Size at Maturation, Spawning Variability and Fecundity in the Queen Conch, Aliger gigas. Gulf and Caribbean Research 31 (1): GCFI 10-GCFI 19. Retrieved from https://aquila.usm.edu/gcr/vol31/iss1/11 DOI: https://doi.org/10.18785/gcr.3101.11 This Gulf and Caribbean Fisheries Institute Partnership is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf and Caribbean Research by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. VOLUME 25 VOLUME GULF AND CARIBBEAN Volume 25 RESEARCH March 2013 TABLE OF CONTENTS GULF AND CARIBBEAN SAND BOTTOM MICROALGAL PRODUCTION AND BENTHIC NUTRIENT FLUXES ON THE NORTHEASTERN GULF OF MEXICO NEARSHORE SHELF RESEARCH Jeffrey G. Allison, M. E. Wagner, M. McAllister, A. K. J. Ren, and R. A. Snyder....................................................................................1—8 WHAT IS KNOWN ABOUT SPECIES RICHNESS AND DISTRIBUTION ON THE OUTER—SHELF SOUTH TEXAS BANKS? Harriet L. Nash, Sharon J. Furiness, and John W. Tunnell, Jr. ......................................................................................................... 9—18 Volume 31 ASSESSMENT OF SEAGRASS FLORAL COMMUNITY STRUCTURE FROM TWO CARIBBEAN MARINE PROTECTED 2020 AREAS ISSN: 2572-1410 Paul A.