2009 Paris, France the Movement Disorder Society’S 13Th International Congress of Parkinson’S Disease and Movement Disorders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Complete Issue
Conerete Bloek & Sprayed Coating- a ~inning eOlDbination of beauty & silDplieity at lo~eost STAND RD CREGO CK WITH CEMENT LE E COAT AND SPRAYED-ON TE U ED INISH COAT • JERRY GOFFE PHOTO RUST TRACTOR COMPANY ELLISON-HAWKINS-VOGT 6' BYRNES, P.A. ARCH ITECTS ·ENGINEERS K. L. HOUSE CONSTRUCTION CO. GENERAL CONTRACTOR KENNETH P. THOMPSON CO., INC. MASONRY CONTRACTOR BILL C. CARROLL CO., INC. SPRAYED COATING "'\ For our reoders I"~, we wish 1976 to \'\'~" be rhapsodic, • thriving, abundant and eudaemonic. , '~-I4 ""_ """""' .----"''''''v~J col: 18 no. 1 jan. - feb. 1976 • new mexico architecture As we begin another year of New Mexico Architecture, it is appropri ate to remind our readers of the contribution made to our financial stability by the advertisers. It is their support which makes possible the production of the magazine. To all of these fine people the "staff" says a most sincere thank you! Space in New Mexico Architecture o DOD as a Resource for on Energy Ethic 10 Beginning on page lOis an arti - By Anthony C. Antoniades, AI.A, AI.P. cle by Anthony C. Antoniades, AlA, AlP, Associate Professor of Archi tecture at the University of Texas at Arlington. Professor Antoniades taught architecture at the Universi ty of New Mexico before moving to Index to Advertisers 18 Texas. It was during those years in our state that he developed a strong interest in and knowledge of the architectural heritage of New Mexi ico. Three articles by Antoniades hove appeared previously in NMA November/December 1971, Septem ber/October 1973 and July/August 1974. -

Xenoport Hurt by Solzira NDA Withdrawal
November 11, 2008 Xenoport hurt by Solzira NDA withdrawal Evaluate Vantage News that Xenoport and partner GlaxoSmithKline’s recent application for restless legs syndrome (RLS) drug Solzira will have to be withdrawn, with the FDA requesting that data from a single trial be re-formatted, caused Xenoport’s shares to fall 13% yesterday to a new 18-month low of $34.44. With a potential 4-6 month delay to approval and the postponement of a $23m milestone that Xenoport was due to receive from Glaxo on the FDA’s acceptance of Solzira’s NDA, senior executives at the specialty US company could be forgiven for feeling somewhat aggrieved with Glaxo for this apparent oversight, given the pharma giant’s supposed experience and expertise in regulatory filings. The setback to Solzira and resulting slump in Xenoport’s share price suggests that shareholders who have not fled the stock will be even more desperate for positive phase IIb data for GERD treatment, XP19986 (Event - XenoPort looking for end of year trial lift, October 10, 2008). Formatting issues Avoiding these kinds of regulatory pitfalls, after all the cost and hard work involved in taking a drug through clinical development, is becoming an increasingly important factor behind a company’s decision to sign up a big pharma partner in today’s tougher regulatory environment. Both companies were naturally keen to stress that the NDA withdrawal has nothing to do with the content of the filing, which probably makes the rejection on a technicality all the more frustrating for Xenoport. Whilst the FDA requested the data from one particular study to be re-formatted, Glaxo has decided to review the formatting of other data sets in the application, presumably to ensure there can be no further grounds for rejection. -

Annual Report
Greeks Helping Greeks ANNUAL REPORT 2019 About THI The Hellenic Initiative (THI) is a global, nonprofi t, secular institution mobilizing the Greek Diaspora and Philhellene community to support sustainable economic recovery and renewal for Greece and its people. Our programs address crisis relief through strong nonprofi t organizations, led by heroic Greeks that are serving their country. They also build capacity in a new generation of heroes, the business leaders and entrepreneurs with the skills and values to promote the long term growth of Hellas. THI Vision / Mission Statement Investing in the future of Greece through direct philanthropy and economic revitalization. We empower people to provide crisis relief, encourage entrepreneurs, and create jobs. We are The Hellenic Initiative (THI) – a global movement of the Greek Diaspora About the Cover Featuring the faces of our ReGeneration Interns. We, the members of the Executive Committee and the Board of Directors, wish to express to all of you, the supporters and friends of The Hellenic Initiative, our deepest gratitude for the trust and support you have given to our organization for the past seven years. Our mission is simple, to connect the Diaspora with Greece in ways which are valuable for Greece, and valuable for the Diaspora. One of the programs you will read about in this report is THI’s ReGeneration Program. In just 5 years since we launched ReGeneration, with the support of the Coca-Cola Co. and the Coca-Cola Foundation and 400 hiring partners, we have put over 1100 people to work in permanent well-paying jobs in Greece. -

Management of Side Effects of Antipsychotics
Management of side effects of antipsychotics Oliver Freudenreich, MD, FACLP Co-Director, MGH Schizophrenia Program www.mghcme.org Disclosures I have the following relevant financial relationship with a commercial interest to disclose (recipient SELF; content SCHIZOPHRENIA): • Alkermes – Consultant honoraria (Advisory Board) • Avanir – Research grant (to institution) • Janssen – Research grant (to institution), consultant honoraria (Advisory Board) • Neurocrine – Consultant honoraria (Advisory Board) • Novartis – Consultant honoraria • Otsuka – Research grant (to institution) • Roche – Consultant honoraria • Saladax – Research grant (to institution) • Elsevier – Honoraria (medical editing) • Global Medical Education – Honoraria (CME speaker and content developer) • Medscape – Honoraria (CME speaker) • Wolters-Kluwer – Royalties (content developer) • UpToDate – Royalties, honoraria (content developer and editor) • American Psychiatric Association – Consultant honoraria (SMI Adviser) www.mghcme.org Outline • Antipsychotic side effect summary • Critical side effect management – NMS – Cardiac side effects – Gastrointestinal side effects – Clozapine black box warnings • Routine side effect management – Metabolic side effects – Motor side effects – Prolactin elevation • The man-in-the-arena algorithm www.mghcme.org Receptor profile and side effects • Alpha-1 – Hypotension: slow titration • Dopamine-2 – Dystonia: prophylactic anticholinergic – Akathisia, parkinsonism, tardive dyskinesia – Hyperprolactinemia • Histamine-1 – Sedation – Weight gain -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

Serotonin Dopamine Antagonists)
NewNew DevelopmentsDevelopments inin thethe ResearchResearch andand TreatmentTreatment ofof SchizophreniaSchizophrenia StephenStephen M.M. Stahl,Stahl, MD,MD, PhDPhD Adjunct Professor, Department of Psychiatry, University of California, San Diego School of Medicine Sponsored by Neuroscience Education Institute Additionally sponsored by American Society for the Advancement of Pharmacotherapy This activity is supported by educational grants from AstraZeneca Pharmaceuticals LP; Cephalon, Inc.; and Shire Pharmaceuticals Inc. with additional support from Alkermes, Inc.; Eli Lilly and Company; Jazz Pharmaceuticals, Inc.; and Sepracor Inc. Copyright © 2007 Neuroscience Education Institute. All rights reserved. Learning Objectives After completion of this lecture you should be able to: • Evaluate evidence about the function and efficacy of the newest antipsychotics • Describe the rationale and present state of pharmacogenetics research • Discuss goals and challenges of pharmacogenetics in psychiatry Copyright © 2008 Neuroscience Education Institute. All rights reserved. Overview: Four Bedtime Stories • A Tale of Serotonin Antagonism • The Dopamine Partial Agonism Story • Pharmacogenetics and the Genes • Once Upon a Glutamate Receptor Copyright © 2008 Neuroscience Education Institute. All rights reserved. Overview: Four Bedtime Stories • A Tale of Serotonin Antagonism • The Dopamine Partial Agonism Story • Pharmacogenetics and the Genes • Once Upon a Glutamate Receptor Copyright © 2008 Neuroscience Education Institute. All rights reserved. What -

Drug-Induced Movement Disorders
Medical Management of Early PD Samer D. Tabbal, M.D. May 2016 Associate Professor of Neurology Director of The Parkinson Disease & Other Movement Disorders Program Mobile: +961 70 65 89 85 email: [email protected] Conflict of Interest Statement No drug company pays me any money Outline So, you diagnosed Parkinson disease .Natural history of the disease .When to start drug therapy? .Which drug to use first for symptomatic treatment? ● Levodopa vs dopamine agonist vs MAOI Natural History of Parkinson Disease Before levodopa: Death within 10 years After levodopa: . “Honeymoon” period (~ 5-7 years) . Motor (ON/OFF) fluctuations & dyskinesias: ● Drug therapy effective initially ● Surgical intervention by 10-15 years - Deep brain stimulation (DBS) therapy Motor Response Dyskinesia 5-7 yrs >10 yrs Dyskinesia ON state ON state OFF state OFF state time time Several days Several hours 1-2 hour Natural History of Parkinson Disease Prominent gait impairment and autonomic symptoms by 20-25 years (Merola 2011) Behavioral changes before or with motor symptoms: . Sleep disorders . Depression . Anxiety . Hallucinations, paranoid delusions Dementia at anytime during the illness . When prominent or early: diffuse Lewy body disease Symptoms of Parkinson Disease Motor Symptoms Sensory Symptoms Mental Symptoms: . Cognitive and psychiatric Autonomic Symptoms Presenting Symptoms of Parkinson Disease Mood disorders: depression and lack of motivation Sleep disorders: “acting out dreams” and nightmares Early motor symptoms: Typically Unilateral . Rest tremor: chin, arms or legs or “inner tremor” . Bradykinesia: focal and generalized slowness . Rigidity: “muscle stiffness or ache” Also: (usually no early postural instability) . Facial masking with hypophonia: “does not smile anymore” or “looks unhappy all the time” . -

Kalamazoo County Naturalization
Kalamazoo County Naturalization Last Name First Name Middle Name First Paper Second Paper Aach Rita Ann V66P5111 Aalbregtse Abraham Peter V10P163 Aalbregtse Johannes V12P446 Aalbregtse Jozias V11P151 Aalbregtse Suzanna Maria V58P3802 Aarssen Diederika Frederika Willemina V13P40 Abbate Santi V13P56 Abbate Santi V34P29 V34P29 Abolins Aina V64P4686 Abolins Augusts V64P4687 Abolins Guntis V64P4688 Abolins Mara V64P4627 Aboshin Aglou Ismail V29P64 V29P64 Abraham Francis T V10P219 Abraham Francis Thurlborn V23P104 V23P104 Abraham Maurice George V10P237 Abraham Thomas J V4P41 Abraham Thomas J V5P134 Abrahamse Pieter V3P262 Abromson Isral V5P314 Achterhof Albert V64P4603 Achterhof Johanna V59P3900 Adam Ben V52P3134 Adam Enke V14P204 Adam Simon V11P102 Adam Simon V14P213 Friday, July 06, 2007 Page 1 of 577 Kalamazoo County Naturalization Index Order copies of records by calling (517) 373-1408 Archives of Michigan Home page: www.michigan.gov/archivesofmi E-mail: [email protected] Last Name First Name Middle Name First Paper Second Paper Adam Simon V35P8 V35P8 Adamopooulos Fotis V34P39 V34P39 Adamopoulos Fotis V14P316 Adamopoulos James V30P27 Adams Aaltise V19P3868 Adams Antje V17P3172 Adams Claus John V51P3022 Adams Ella Nettie V71P27 Adams Enke V18P3596 Adams Frank V14P316 Adams Frank V34P39 V34P39 Adams Jacoba V57P3649 Adams James V30P27 Adams Kaert V46P2556 Adams Koert V46P2556 Adams Magdalena Anne V51P3034 Adams Nicky V58P3833 Adkin Marion Jean V53P3215 Adler Nathan V52P3189 Advocaat John Edward V21P4397 Aelick William John V23P142 V23P142 -

Substituted 3-Isobutyl-9, 10-Dimethoxy-1,3,4,6,7,11B
(19) TZZ Z_ 9B_T (11) EP 2 081 929 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 471/04 (2006.01) 09.01.2013 Bulletin 2013/02 (86) International application number: (21) Application number: 07864160.2 PCT/US2007/084176 (22) Date of filing: 08.11.2007 (87) International publication number: WO 2008/058261 (15.05.2008 Gazette 2008/20) (54) SUBSTITUTED 3-ISOBUTYL-9, 10-DIMETHOXY-1,3,4,6,7,11B-HEXAHYDRO-2H-PYRIDO[2,1-A] ISOQUINOLIN-2-OL COMPOUNDS AND METHODS RELATING THERETO SUBSTITUIERTE 3-ISOBUTYL-9,10-DIMETHOXY-1,3,4,6,7,11B-HEXAHYDRO-2H-PYRIDO[2,1-A] ISOCHINOLIN-2-OLVERBINDUNGEN UND DIESE BETREFFENDE VERFAHREN COMPOSÉS 3-ISOBUTYL-9, 10-DIMÉTHOXY-1,3,4,6,7,11B-HEXAHYDRO-2H-PYRIDO[2,1-A] ISOQUINOLIN-2-OL SUBSTITUÉS ET PROCÉDÉS ASSOCIÉS (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • BROSSIA ET AL: "SYNTHESEVERSUCHE IN DER HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE EMETIN-REIHE 3. MITTEILUNG 2-HYDROXY- SI SK TR HYDROBENZOÄAÜCHINOLIZINE" HELVETICA Designated Extension States: CHIMICA ACTA, VERLAG HELVETICA CHIMICA AL BA HR MK RS ACTA. BASEL, CH, vol. 41, no. 4, 1958, pages 1793-1806, XP008047475 ISSN: 0018-019X (30) Priority: 08.11.2006 US 864944 P • KILBOURN M R ET AL: "Absolute Configuration of (+)-alpha-Dihydrotetrabenazine, an Active (43) Date of publication of application: Metabolite of Tetrabenazine" CHIRALITY, WILEY- 29.07.2009 Bulletin 2009/31 LISS, NEW YORK, US, vol. -

Tetrabenazine: the First Approved Drug for the Treatment of Chorea in US Patients with Huntington Disease
Neuropsychiatric Disease and Treatment Dovepress open access to scientific and medical research Open Access Full Text Article REVIEW 7HWUDEHQD]LQHWKHÀUVWDSSURYHGGUXJ for the treatment of chorea in US patients with Huntington disease Samuel Frank Abstract: Huntington disease (HD) is a dominantly inherited progressive neurological disease Boston University School of Medicine, characterized by chorea, an involuntary brief movement that tends to flow between body regions. Boston, Massachusetts, USA HD is typically diagnosed based on clinical findings in the setting of a family history and may be confirmed with genetic testing. Predictive testing is available to those at risk, but only experienced clinicians should perform the counseling and testing. Multiple areas of the brain degenerate mainly involving the neurotransmitters dopamine, glutamate, and G-aminobutyric acid. Although pharmacotherapies theoretically target these neurotransmitters, few well-conducted trials for symptomatic or neuroprotective interventions yielded positive results. Tetrabenazine (TBZ) is a dopamine-depleting agent that may be one of the more effective agents for reducing chorea, although it has a risk of potentially serious adverse effects. Some newer antipsychotic agents, such as olanzapine and aripiprazole, may have adequate efficacy with a more favorable adverse-effect profile than older antipsychotic agents for treating chorea and psychosis. This review will address the epidemiology and diagnosis of HD as background for understanding potential pharmacological treatment options. Because TBZ is the only US Food and Drug Administration-approved medication in the United States for HD, the focus of this review will be on its pharmacology, efficacy, safety, and practical uses. There are no current treatments to change the course of HD, but education and symptomatic therapies can be effective tools for clinicians to use with patients and families affected by HD. -
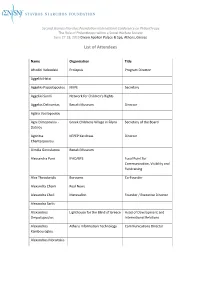
List of Attendees
Second Stavros Niarchos Foundation International Conference on Philanthropy The Role of Philanthropy within a Social Welfare Society June 27-28, 2013 Divani Apollon Palace & Spa, Athens, Greece List of Attendees Name Organization Title Afroditi Veloudaki Prolepsis Program Director Aggeliki Hatzi Aggeliki Papadopoulou KIKPE Secretary Aggeliki Sandi Network for Children's Rights Aggelos Delivorrias Benaki Museum Director Aglaia Vasilopoulou Agni Dimopoulou - Greek Childrens Village in Filyro Secretary of the Board Datsiou Agoritsa KEPEP Karditsas Director Chantzopoulou Aimilia Geroulanou Benaki Museum Alessandra Pani IFAD/BFS Focal Point for Communication, Visibility and Fundraising Alex Theodoridis Boroume Co-Founder Alexandra Chaini Real News Alexandra Choli Metavallon Founder / Executive Director Alexandra Sarlis Alexandros Lighthouse for the Blind of Greece Head of Development and Despotopoulos International Relations Alexandros Athens Information Technology Communications Director Kambouroglou Alexandros Moraitakis Name Organization Title Alexandros Taxildaris Association for People with President Mobility Problems and Friends Perpato Alexia Divani Alexia Kotsopoulou AWOG Representative Alexia Raphael Stavros Niarchos Foundation Intern Aliki Martinou Mazigia to Paidi Aliki Mitsakou Aliki Tserketzoglou Galilee Palliative Care Unit Amalia Delicari Stavros Niarchos Foundation Associate Program Officer Amalia Zeppou Municipality of Athens Amvrosios Holy Metropolis of Kalavryta and Metropolitan Bishop Aegialia Anastasia Andritsou British