The Immunoproteasome in Antigen Processing and Other
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antigen Presentation by Dendritic Cells and Their Significance in Antineoplastic Immunotherapy
in vivo 18: 81-100 (2004) Antigen Presentation by Dendritic Cells and their Significance in Antineoplastic Immunotherapy BELA BODEY1,2, STUART E. SIEGEL1,2 and HANS E. KAISER3 1Department of Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA; 2Childrens’ Center for Cancer and Blood Diseases, Childrens’ Hospital Los Angeles, Los Angeles, CA; 3Department of Pathology, School of Medicine, University of Maryland, Baltimore, MD, U.S.A. and Department of General and Experimental Pathology, University of Vienna, Vienna, Austria Abstract. Dendritic cells (DCs) are present in essentially every derived peptides on the surface of major histocompatibility mammalian tissue, where they operate at the interface of innate complex (MHC) molecules and acquire the cellular specialization and acquired immunity by recognizing pathogens and presenting to select and activate naive antigen-specific T lymphocytes. pathogen-derived peptides to T lymphocytes. According to the Immunotherapeutic ideas are based on the ability of the research group of Shortman (1-9), experimental results suggest a mammalian immune system to recognize neoplastically "dual" DC differentiation model, demonstrating the existence of transformed cells. Immunotherapy of human neoplasms has both myeloid-derived (with characteristic IF: CD11b+, CD11c+, always represented a very attractive fourth-modality therapeutic CD8alpha- and DEC205+) and lymphoid-derived DCs (showing approach, especially in light of the many shortcomings of CD11b-, CD11c-, CD8alpha+ and DEC205+ IF). DCs, including conventional surgical, radiation and chemotherapies in the interdigitating cells (IDCs) and Langerhans cells (LCs), are management of neoplastically transformed cells. The cancer characterized by dendritic morphology, high migratory mobility and vaccine approach to therapy is based on the notion that the are the most effective, "professional" cells for antigen presentation immune system could possibly mount a rejection strength response in primary immune responses. -

An Integrative Analysis of Gene Expression and Molecular
Muraro and Simmons BMC Bioinformatics (2016) 17:42 DOI 10.1186/s12859-016-0886-z RESEARCH ARTICLE Open Access An integrative analysis of gene expression and molecular interaction data to identify dys-regulated sub-networks in inflammatory bowel disease Daniele Muraro* and Alison Simmons Abstract Background: Inflammatory bowel disease (IBD) consists of two main disease-subtypes, Crohn’s disease (CD) and ulcerative colitis (UC); these subtypes share overlapping genetic and clinical features. Genome-wide microarray data enable unbiased documentation of alterations in gene expression that may be disease-specific. As genetic diseases are believed to be caused by genetic alterations affecting the function of signalling pathways, module-centric optimisation algorithms, whose aim is to identify sub-networks that are dys-regulated in disease, are emerging as promising approaches. Results: In order to account for the topological structure of molecular interaction networks, we developed an optimisation algorithm that integrates databases of known molecular interactions with gene expression data; such integration enables identification of differentially regulated network modules. We verified the performance of our algorithm by testing it on simulated networks; we then applied the same method to study experimental data derived from microarray analysis of CD and UC biopsies and human interactome databases. This analysis allowed the extraction of dys-regulated subnetworks under different experimental conditions (inflamed and uninflamed tissues in CD and UC). Optimisation was performed to highlight differentially expressed network modules that may be common or specific to the disease subtype. Conclusions: We show that the selected subnetworks include genes and pathways of known relevance for IBD; in particular, the solutions found highlight cross-talk among enriched pathways, mainly the JAK/STAT signalling pathway and the EGF receptor signalling pathway. -

Antigen Processing (Major Hbitocompatlbility Complex/Class I Moecules/Lymphokines) YOUNG YANG, JAMES B
Proc. Nati. Acad. Sci. USA Vol. 89, pp. 4928-4932, June 1992 Immunology Proteasomes are regulated by interferon y: Implications for antigen processing (major hbitocompatlbility complex/class I moecules/lymphokines) YOUNG YANG, JAMES B. WATERS, KLAUS FROH, AND PER A. PETERSON Department of Immunology, The Scripps Research Institute, La Jolla, CA 92037 Communicated by Frank J. Dixon, February 27, 1992 ABSTRACT Class I major histocompatibility complex strengthened by the findings, presented in this communica- (MHC) molecules present antigenic peptides of cytoplasmic tion, that several proteasomal subunits, including MHC- origin to T cells. As the lengths ofthese peptides seem stried encoded subunits, are regulated by interferon y (IFN--y) and to eight or nine amino acids, an unusual proteolytic system that the incorporation of several more subunits into protea- must play a role in antigen processing. Proteasomes, a major somes appears to depend on the expression of the MHC- extralysosomal proteolytic system, are responsible for the encoded proteasomal subunits. Moreover, the pattern of degradation of cytoplasmic proteins. We demonstrate that expression of IFN-y-regulated subunits suggests complexi- several proteasomal subunits, including MHC-encoded sub- ties in the regulation of proteasomes with respect to its units, are regulated by interferon y. These data and the finding subunit composition, subcellular localization, and its incor- that MHC-encoded and other interferon -regulated protea- poration into larger ubiquitin-related proteolytic complexes. somal subunits are uniquely associated with proteasomes Possible functions for the MHC-encoded and IFN-y- strongly suggest that the immune system has recruited protea- regulated proteasomal subunits in antigen processing are somes for antigen processing. -

Cytoplasmic Proteolysis Antigens on MHC Class II Is Dependent On
Efficient Presentation of Both Cytosolic and Endogenous Transmembrane Protein Antigens on MHC Class II Is Dependent on Cytoplasmic Proteolysis This information is current as of September 28, 2021. Paushali Mukherjee, Aadish Dani, Sumeena Bhatia, Nagendra Singh, Alexander Y. Rudensky, Anna George, Vineeta Bal, Satyajit Mayor and Satyajit Rath J Immunol 2001; 167:2632-2641; ; doi: 10.4049/jimmunol.167.5.2632 Downloaded from http://www.jimmunol.org/content/167/5/2632 References This article cites 51 articles, 20 of which you can access for free at: http://www.jimmunol.org/content/167/5/2632.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 28, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2001 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Efficient Presentation of Both Cytosolic and Endogenous Transmembrane Protein Antigens on MHC Class II Is Dependent on Cytoplasmic Proteolysis1 Paushali Mukherjee,* Aadish Dani,† Sumeena Bhatia,* Nagendra Singh,* Alexander Y. -

On the Role of the Immunoproteasome in Transplant Rejection
Immunogenetics (2019) 71:263–271 https://doi.org/10.1007/s00251-018-1084-0 REVIEW On the role of the immunoproteasome in transplant rejection Michael Basler1,2 & Jun Li1,3 & Marcus Groettrup1,2 Received: 17 July 2018 /Accepted: 4 September 2018 /Published online: 15 September 2018 # Springer-Verlag GmbH Germany, part of Springer Nature 2018 Abstract The immunoproteasome is expressed in cells of hematopoietic origin and is induced during inflammation by IFN-γ. Targeting the immunoproteasome with selective inhibitors has been shown to be therapeutically effective in pre-clinical models for autoim- mune diseases, colitis-associated cancer formation, and transplantation. Immunoproteasome inhibition prevents activation and proliferation of lymphocytes, lowers MHC class I cell surface expression, reduces the expression of cytokines of activated immune cells, and curtails T helper 1 and 17 cell differentiation. This might explain the in vivo efficacy of immunoproteasome inhibition in different pre-clinical disease models for autoimmunity, cancer, and transplantation. In this review, we summarize the effect of immunoproteasome inhibition in different animal models for transplantation. Keywords Proteasome . Immunoproteasome . Antigen processing . Antigen presentation . Transplantation Introduction et al. 2012). Depending on the cell type and the presence or absence of the pro-inflammatory cytokine interferon (IFN)-γ, The proteasome is responsible for the degradation of proteins the three inducible β subunits of the immunoproteasome low in the cytoplasm and nuclei of all eukaryotic cells and exerts molecular mass polypeptide (LMP)2 (β1i), multicatalytic en- numerous essential regulatory functions in nearly all cell bio- dopeptidase complex-like (MECL)-1 (β2i), and LMP7 (β5i), logical pathways. The 26S proteasome degrades poly- can, in addition to the corresponding constitutive subunits ubiquitylated protein substrates and consists of a 19S regulator β1c, β2c, and β5c, enrich the cellular assortment of catalyti- and a 20S proteolytic core complex. -

Protein Expression Analysis of an in Vitro Murine Model of Prostate Cancer Progression: Towards Identification of High-Potential Therapeutic Targets
Journal of Personalized Medicine Article Protein Expression Analysis of an In Vitro Murine Model of Prostate Cancer Progression: Towards Identification of High-Potential Therapeutic Targets Hisham F. Bahmad 1,2,3 , Wenjing Peng 4, Rui Zhu 4, Farah Ballout 1, Alissar Monzer 1, 1,5 6, , 1, , 4, , Mohamad K. Elajami , Firas Kobeissy * y , Wassim Abou-Kheir * y and Yehia Mechref * y 1 Department of Anatomy, Cell Biology and Physiological Sciences, Faculty of Medicine, American University of Beirut, Beirut 1107-2020, Lebanon; [email protected] (H.F.B.); [email protected] (F.B.); [email protected] (A.M.); [email protected] (M.K.E.) 2 Arkadi M. Rywlin M.D. Department of Pathology and Laboratory Medicine, Mount Sinai Medical Center, Miami Beach, FL 33140, USA 3 Herbert Wertheim College of Medicine, Florida International University, Miami, FL 33199, USA 4 Department of Chemistry and Biochemistry, Texas Tech University, Lubbock, TX 79409, USA; [email protected] (W.P.); [email protected] (R.Z.) 5 Department of Internal Medicine, Mount Sinai Medical Center, Miami Beach, FL 33140, USA 6 Department of Biochemistry and Molecular Genetics, Faculty of Medicine, American University of Beirut, Beirut 1107-2020, Lebanon * Correspondence: [email protected] (F.K.); [email protected] (W.A.-K.); [email protected] (Y.M.); Tel.: +961-1-350000 (ext. 4805) (F.K.); +961-1-350000 (ext. 4778) (W.A.K.); +1-806-834-8246 (Y.M.); Fax: +1-806-742-1289 (Y.M.); 961-1-744464 (W.A.K.) These authors have contributed equally to this work as joint senior authors. -
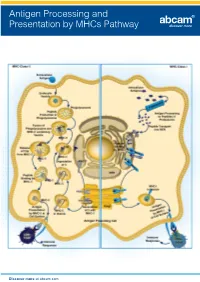
Antigen Processing and Presentation by Mhcs Pathway
Antigen Processing and Presentation by MHCs Pathway Discover more at abcam.com Antigen Processing and Presentation by MHCs Pathway Related antibodies Product This image shows MHC Class II highlights Product Clonality Applications Host Species Reactivity Product code expressing cells in formalin fixed and paraffin embedded human lymph MHC class I antibody [EP1395Y] M Flow Cyt, ICC/IF, IHC-P, IP, WB Rb Hu, Ms, Rat 52922 node, using ab55152. MHC class I antibody [AF6-88.5.5.3] (Biotin) M Flow Cyt Ms Ms 93528 MHC Class II antibody MHC class I antibody [34-1-2S] (FITC) M Flow Cyt Ms Ms 95572 (ab55152) MHC class I antibody [34-1-2S] (Phycoerythrin) M Flow Cyt Ms Ms 95571 MHC Class I alpha antibody [F21-2] M AP, Flow Cyt, IP, WB Ms Chk 23483 Clonality Applications MHC Class 1 H2 Db antibody [27-11-13] - BSA and Azide free M Flow Cyt, FuncS, IHC-Fr Ms Ms 25244 M IHC-P, WB MHC Class 1 H2 Db antibody [28-14-8] (FITC) M Flow Cyt Ms Ms 25056 Host Species cross reactivity MHC Class 1 H2 Db antibody [KH95] M Flow Cyt, FuncS, WB Ms Ms 64373 Ms Hu, RecFrag MHC Class I H2 Dd antibody [34-2-12] M Flow Cyt, FuncS, IHC-Fr, IP Ms Ms 64368 MHC Class I H2 Dk antibody [15-5-5.3] M Flow Cyt Ms Ms 25216 MHC Class II antigens are a valuable tool for studying T helper cell interactions with class II MHC Class I H2 Kb antibody P ELISA, WB Rb Ms 93364 positive antigen presenting cells, as well as the MHC Class I H2 Kd + Dd + q + u + v antibody [1.B.552] (FITC) M Flow Cyt, IP Ms Ms 62228 development of T helper cells since the antigen is present on stromal cells in the thymus. -

Molecular Pathology and Novel Clinical Therapy for Uterine
ANTICANCER RESEARCH 36 : 4997-5008 (2016) doi:10.21873/anticanres.11068 Review Molecular Pathology and Novel Clinical Therapy for Uterine Leiomyosarcoma TAKUMA HAYASHI 1,2 , MIKI KAWANO 2,3 , TOMOYUKI ICHIMURA 4, KOICHI IDA 1, HIROFUMI ANDO 1, DORIT ZHARHARY 5, YAE KANAI 6, HIROYUKI ABURATANI 7, SUSUMU TONEGAWA 8, TANRI SHIOZAWA 1, NOBUO YAEGASHI 9 and IKUO KONISHI 10 1Department of Obstetrics and Gynecology, Shinshu University School of Medicine, Nagano, Japan; 2Department of Medical Technology, International University of Health and Welfare, Chiba, Japan; 3Department of Health Science, Kyushu University Graduate School of Medicine, Fukuoka, Japan; 4Department of Obstetrics and Gynecology, Osaka City University Graduate School of Medicine, Osaka, Japan; 5SIGMA-Aldrich Israel, Rehovot, Israel; 6Pathology Division, Keio University School of Medicine, Tokyo, Japan; 7The Cancer System Laboratory, Research Center for Advanced Science and Technology, The University of Tokyo, Tokyo, Japan; 8Department of Biology, Massachusetts Institute of Technology, Cambridge, MA, U.S.A.; 9Department of Obstetrics and Gynecology, Tohoku University Graduate School of Medicine, Miyagi, Japan; 10 National Hospital Organization Kyoto Medical Centre, Kyoto, Japan Abstract. Patients with uterine leiomyosarcoma (LMS) disease prevalence of ~37% by 12 months of age. Furthermore, typically present with vaginal bleeding, pain, and a pelvic a recent report showed the loss of ability to induce PSMB9/ β1 i mass, with atypical presentations of hypercalcemia and expression, -

Datasheet: VPA00155 Product Details
Datasheet: VPA00155 Description: GOAT ANTI PSMB8 Specificity: PSMB8 Format: Purified Product Type: PrecisionAb™ Polyclonal Isotype: Polyclonal IgG Quantity: 100 µl Product Details Applications This product has been reported to work in the following applications. This information is derived from testing within our laboratories, peer-reviewed publications or personal communications from the originators. Please refer to references indicated for further information. For general protocol recommendations, please visit www.bio-rad-antibodies.com/protocols. Yes No Not Determined Suggested Dilution Western Blotting 1/1000 PrecisionAb antibodies have been extensively validated for the western blot application. The antibody has been validated at the suggested dilution. Where this product has not been tested for use in a particular technique this does not necessarily exclude its use in such procedures. Further optimization may be required dependant on sample type. Target Species Human Species Cross Reacts with: Rat Reactivity N.B. Antibody reactivity and working conditions may vary between species. Product Form Purified IgG - liquid Preparation Goat polyclonal antibody purified by affinity chromatography Buffer Solution TRIS buffered saline Preservative 0.02% Sodium Azide (NaN ) 0.5 % BSA Stabilisers 3 Immunogen Peptide with the sequence C-DVSDLLHQYREANQ, from the C terminus of the protein sequence External Database Links UniProt: P28062 Related reagents Entrez Gene: 5696 PSMB8 Related reagents Synonyms LMP7, PSMB5i, RING10, Y2 Page 1 of 2 Specificity Goat anti Human PSMB8 antibody recognizes proteasome subunit beta type-8, also known as Low molecular mass protein 7, macropain subunit C13, multicatalytic endopeptidase complex subunit C13, proteasome component C13, and proteasome subunit beta-5i. The proteasome is a multicatalytic proteinase complex with a highly ordered ring-shaped 20S core structure. -

Proteasome Immunosubunits Protect Against the Development of CD8 T Cell-Mediated Autoimmune Diseases
Proteasome Immunosubunits Protect against the Development of CD8 T Cell-Mediated Autoimmune Diseases This information is current as Dietmar M. W. Zaiss, Cornelis P. J. Bekker, Andrea Gröne, of September 29, 2021. Benedicte A. Lie and Alice J. A. M. Sijts J Immunol published online 29 July 2011 http://www.jimmunol.org/content/early/2011/07/29/jimmun ol.1101003 Downloaded from Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision http://www.jimmunol.org/ • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: by guest on September 29, 2021 http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2011 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Published July 29, 2011, doi:10.4049/jimmunol.1101003 The Journal of Immunology Proteasome Immunosubunits Protect against the Development of CD8 T Cell-Mediated Autoimmune Diseases Dietmar M. W. Zaiss,* Cornelis P. J. Bekker,* Andrea Gro¨ne,† Benedicte A. Lie,‡ and Alice J. A. M. Sijts* Exposure of cells to inflammatory cytokines induces the expression of three proteasome immunosubunits, two of which are encoded in the MHC class II region. -

The Role of the Immunoproteasome in Interferon-Γ-Mediated Microglial Activation Received: 4 May 2017 Kasey E
www.nature.com/scientificreports OPEN The role of the immunoproteasome in interferon-γ-mediated microglial activation Received: 4 May 2017 Kasey E. Moritz1, Nikki M. McCormack1, Mahlet B. Abera2, Coralie Viollet3, Young J. Yauger1, Accepted: 14 July 2017 Gauthaman Sukumar3, Clifton L. Dalgard1,2,3,4 & Barrington G. Burnett 1,2 Published: xx xx xxxx Microglia regulate the brain microenvironment by sensing damage and neutralizing potentially harmful insults. Disruption of central nervous system (CNS) homeostasis results in transition of microglia to a reactive state characterized by morphological changes and production of cytokines to prevent further damage to CNS tissue. Immunoproteasome levels are elevated in activated microglia in models of stroke, infection and traumatic brain injury, though the exact role of the immunoproteasome in neuropathology remains poorly defned. Using gene expression analysis and native gel electrophoresis we characterize the expression and assembly of the immunoproteasome in microglia following interferon-gamma exposure. Transcriptome analysis suggests that the immunoproteasome regulates multiple features of microglial activation including nitric oxide production and phagocytosis. We show that inhibiting the immunoproteasome attenuates expression of pro-infammatory cytokines and suppresses interferon-gamma-dependent priming of microglia. These results imply that targeting immunoproteasome function following CNS injury may attenuate select microglial activity to improve the pathophysiology of neurodegenerative conditions or the progress of infammation-mediated secondary injury following neurotrauma. Microglia are the primary infammatory mediators of the central nervous system (CNS). Damage to the CNS results in the transition of microglia from a surveying or ‘ramifed’ state, to a ‘reactive’ state, allowing them to respond to changes in the local milieu1–3. -

Intratumoral Injection of SYNB1891, a Synthetic Biotic Medicine Designed
Intratumoral injection of SYNB1891 A Synthetic Biotic medicine designed to activate the innate immune system. Therapy demonstrates target engagement in humans including intratumoral STING activation. Janku F, MD Anderson Cancer Center; Luke JJ, UPMC Hillman Cancer Center; Brennan AM, Synlogic; Riese RJ, Synlogic; Varterasian M, Pharmaceutical Consultant; Kuhn K, Synlogic; Sokolovska A, Synlogic; Strauss J, Mary Crowley Cancer Research Presented by Filip Janku, MD, PhD Study supported by Synlogic, Inc American Association for Cancer Research (AACR) April 2021 Introduction and Methods SYNB1891 Strain Phase 1 First-in-Human Clinical Trial • Live, modified strain of the probiotic E. coli • Enrolling patients with refractory advanced solid Nissle engineered to produce cyclic tumors or lymphoma dinucleotides (CDN) under hypoxia leading to stimulator of interferon genes (STING)- • Intratumoral (IT) injection of SYNB1891 on Days activation 1, 8 and 15 of the first 21-day cycle and then on Day 1 of each subsequent cycle. • Preferentially taken up by phagocytic antigen- presenting cells in tumors, activating • Dose escalation planned across 7 cohorts (1x106 complementary innate immune pathways – 1x109 live cells) with Arm 1 consisting of (direct CDN STING activation; cGAS-mediated SYNB1891 as monotherapy, and Arm 2 in STING activation and TLR4/MyD88 activation by combination with atezolizumab the bacterial chassis) SYNB1891 was safe and well-tolerated in heterogenous population Nov 2020: Interim Analysis IA Updated through 15 Mar 2021 15 Mar 2021: