Tetramethyldisiloxane: a Practical Organosilane Reducing Agent Jaan Pesti and Gerald L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Reaction of Aminonitriles with Aminothiols: a Way to Thiol-Containing Peptides and Nitrogen Heterocycles in the Primitive Earth Ocean
life Article The Reaction of Aminonitriles with Aminothiols: A Way to Thiol-Containing Peptides and Nitrogen Heterocycles in the Primitive Earth Ocean Ibrahim Shalayel , Seydou Coulibaly, Kieu Dung Ly, Anne Milet and Yannick Vallée * Univ. Grenoble Alpes, CNRS, Département de Chimie Moléculaire, Campus, F-38058 Grenoble, France; [email protected] (I.S.); [email protected] (S.C.); [email protected] (K.D.L.); [email protected] (A.M.) * Correspondence: [email protected] Received: 28 September 2018; Accepted: 18 October 2018; Published: 19 October 2018 Abstract: The Strecker reaction of aldehydes with ammonia and hydrogen cyanide first leads to α-aminonitriles, which are then hydrolyzed to α-amino acids. However, before reacting with water, these aminonitriles can be trapped by aminothiols, such as cysteine or homocysteine, to give 5- or 6-membered ring heterocycles, which in turn are hydrolyzed to dipeptides. We propose that this two-step process enabled the formation of thiol-containing dipeptides in the primitive ocean. These small peptides are able to promote the formation of other peptide bonds and of heterocyclic molecules. Theoretical calculations support our experimental results. They predict that α-aminonitriles should be more reactive than other nitriles, and that imidazoles should be formed from transiently formed amidinonitriles. Overall, this set of reactions delineates a possible early stage of the development of organic chemistry, hence of life, on Earth dominated by nitriles and thiol-rich peptides (TRP). Keywords: origin of life; prebiotic chemistry; thiol-rich peptides; cysteine; aminonitriles; imidazoles 1. Introduction In ribosomes, peptide bonds are formed by the reaction of the amine group of an amino acid with an ester function. -
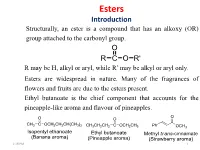
Esters Introduction Structurally, an Ester Is a Compound That Has an Alkoxy (OR) Group Attached to the Carbonyl Group
Esters Introduction Structurally, an ester is a compound that has an alkoxy (OR) group attached to the carbonyl group. O R C O R' R may be H, alkyl or aryl, while R’ may be alkyl or aryl only. Esters are widespread in nature. Many of the fragrances of flowers and fruits are due to the esters present. Ethyl butanoate is the chief component that accounts for the pineapple-like aroma and flavour of pineapples. 1:18 PM 1 Nomenclature of Esters Names of esters consist of two words that reflect the composite structure of the ester. The first word is derived from the alkyl group of the alcohol component, and the second word from the carboxylate group of the carboxylic acid component of the ester. The name of the carboxylate portion is derived by substituting the -ic acid suffix of the parent carboxylic acid with the –ate suffix. The alkyl group is cited first followed by the carboxylate group separated by a space. An ester is thus named as an alkyl 1:18 PM alkanoate. 2 IUPAC Nomenclature of Esters Examples 1:18 PM 3 Synthesis of Esters Preparative Strategies Highlighted below are some of the most common strategies by which esters are prepared. The esters are commonly prepared from the reaction of carboxylic acids, acid chlorides and acid anhydrides with alcohols. 1:18 PM 4 Synthesis of Esters Acid-Catalysed Esterification of a Carboxylic Acid and an Alcohol The acid-catalysed reaction of carboxylic acids and alcohols provides esters. Typically, a catalytic amount of a strong inorganic (mineral) acid such as H2SO4, HCl and H3PO4 is used. -

Hydrogen Atom Transfer-Mediated Cyclisations of Nitriles
Hydrogen Atom Transfer-Mediated Cyclisations of Nitriles Oliver J. Turner,*[a,b] John A. Murphy,*[b] David. J. Hirst[a] and Eric P. A. Talbot*[a]† Abstract: Hydrogen atom transfer-mediated intramolecular C-C coupling reactions between alkenes and nitriles, using PhSiH3 and catalytic Fe(acac)3, are described. This introduces a new strategic bond disconnection for ring-closing reactions, forming ketones via imine intermediates. Of note is the scope of the reaction, including formation of sterically hindered ketones, spirocycles and fused cyclic systems. In the early 1960s, Kwiatek and Seyler first reported the use of metal hydrides as catalysts in the hydrogenation of α,β- unsaturated compounds.[1,2] The discovery by Halpern,[3] later elegantly developed by Norton,[4] that metal-hydride hydrogen atom transfer (HAT) proceeded by a free-radical mechanism opened the door to a wide range of alkene hydrofunctionalisation reactions. But it was the pioneering work by Mukaiyama[5] on the catalytic hydration of alkenes, using Co(acac)2 and oxygen, that sparked wider interest in the field of alkene hydrofunctionalisation. As a result, there now exists an extensive ‘toolkit’ for the addition of hydrogen and a functional group to an alkene with Markovnikov selectivity and high chemo-selectivity using cobalt, manganese and iron complexes.[6,7] Efforts have also been made to extend HAT methodologies to C-C bond formation, both in an intra- and intermolecular fashion: Baran’s group developed a general C-C coupling reaction, utilising electron-deficient alkenes as capable radical acceptors (Scheme 1ai).[8–10] Hydropyridylation of alkenes by intramolecular Minisci reaction was recently demonstrated by Starr,[11] which allows for the formation of structures such as Scheme 1. -

Nitrile Synthesis with Aldoxime Dehydratases: a Biocatalytic Platform with Applications in Asymmetric Synthesis, Bulk Chemicals, and Biorefineries
molecules Review Nitrile Synthesis with Aldoxime Dehydratases: A Biocatalytic Platform with Applications in Asymmetric Synthesis, Bulk Chemicals, and Biorefineries Pablo Domínguez de María Sustainable Momentum, SL, Av. Ansite 3, 4–6, 35011 Las Palmas de Gran Canaria, Canary Islands, Spain; [email protected]; Tel.: +34-6-0956-5237 Abstract: Nitriles comprise a broad group of chemicals that are currently being industrially produced and used in fine chemicals and pharmaceuticals, as well as in bulk applications, polymer chemistry, solvents, etc. Aldoxime dehydratases catalyze the cyanide-free synthesis of nitriles starting from aldoximes under mild conditions, holding potential to become sustainable alternatives for industrial processes. Different aldoxime dehydratases accept a broad range of aldoximes with impressive high substrate loadings of up to >1 Kg L−1 and can efficiently catalyze the reaction in aqueous media as well as in non-aqueous systems, such as organic solvents and solvent-free (neat substrates). This paper provides an overview of the recent developments in this field with emphasis on strategies that may be of relevance for industry and sustainability. When possible, potential links to biorefineries and to the use of biogenic raw materials are discussed. Keywords: biocatalysis; green chemistry; nitriles; aldoxime dehydratases; sustainability Citation: Domínguez de María, P. Nitrile Synthesis with Aldoxime Dehydratases: A Biocatalytic Platform with Applications in Asymmetric Synthesis, Bulk 1. Aldoxime Dehydratases as Biocatalysts for the Cyanide-Free Synthesis of Nitriles Chemicals, and Biorefineries. Nitriles comprise an important group of chemicals that are widely spread in industry Molecules 2021, 26, 4466. in a broad range of sectors, being used as products, solvents, polymers, commodities, or https://doi.org/10.3390/ as starting materials for the production of other chemicals such as amines, amides, etc. -

Fermentation and Ester Taints
Fermentation and Ester Taints Anita Oberholster Introduction: Aroma Compounds • Grape‐derived –provide varietal distinction • Yeast and fermentation‐derived – Esters – Higher alcohols – Carbonyls – Volatile acids – Volatile phenols – Sulfur compounds What is and Esters? • Volatile molecule • Characteristic fruity and floral aromas • Esters are formed when an alcohol and acid react with each other • Few esters formed in grapes • Esters in wine ‐ two origins: – Enzymatic esterification during fermentation – Chemical esterification during long‐term storage Ester Formation • Esters can by formed enzymatically by both the plant and microbes • Microbes – Yeast (Non‐Saccharomyces and Saccharomyces yeast) – Lactic acid bacteria – Acetic acid bacteria • But mainly produced by yeast (through lipid and acetyl‐CoA metabolism) Ester Formation Alcohol function Keto acid‐Coenzyme A Ester Ester Classes • Two main groups – Ethyl esters – Acetate esters • Ethyl esters = EtOH + acid • Acetate esters = acetate (derivative of acetic acid) + EtOH or complex alcohol from amino acid metabolism Ester Classes • Acetate esters – Ethyl acetate (solvent‐like aroma) – Isoamyl acetate (banana aroma) – Isobutyl acetate (fruit aroma) – Phenyl ethyl acetate (roses, honey) • Ethyl esters – Ethyl hexanoate (aniseed, apple‐like) – Ethyl octanoate (sour apple aroma) Acetate Ester Formation • 2 Main factors influence acetate ester formation – Concentration of two substrates acetyl‐CoA and fusel alcohol – Activity of enzyme responsible for formation and break down reactions • Enzyme activity influenced by fermentation variables – Yeast – Composition of fermentation medium – Fermentation conditions Acetate/Ethyl Ester Formation – Fermentation composition and conditions • Total sugar content and optimal N2 amount pos. influence • Amount of unsaturated fatty acids and O2 neg. influence • Ethyl ester formation – 1 Main factor • Conc. of precursors – Enzyme activity smaller role • Higher fermentation temp formation • C and N increase small effect Saerens et al. -

Reduction of Organic Functional Groups Using Hypophosphites Rim Mouselmani
Reduction of Organic Functional Groups Using Hypophosphites Rim Mouselmani To cite this version: Rim Mouselmani. Reduction of Organic Functional Groups Using Hypophosphites. Other. Univer- sité de Lyon; École Doctorale des Sciences et de Technologie (Beyrouth), 2018. English. NNT : 2018LYSE1241. tel-02147583v2 HAL Id: tel-02147583 https://tel.archives-ouvertes.fr/tel-02147583v2 Submitted on 5 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THESE de DOCTORAT DE L’UNIVERSITE DE LYON EN COTUTELLE AVEC L'UNIVERSITÉ LIBANAISE opérée au sein de l’Université Claude Bernard Lyon 1 École Doctorale de Chimie-École Doctorale des Sciences et Technologies Discipline : Chimie Soutenue publiquement le 07/11/2018, par Rim MOUSELMANI Reduction of Organic Functional Groups Using Hypophosphites Devant le jury composé de Mme. Micheline DRAYE Université Savoie Mont Blanc Rapporteure M. Mohammad ELDAKDOUKI Université Arabe de Beyrouth Rapporteur Mme. Emmanuelle SCHULZ Université Paris 11 examinatrice M. Abderrahmane AMGOUNE Université Lyon 1 Président M. Mahmoud FARAJ Université Internationale Libanaise examinateur Mme. Estelle MÉTAY Université Lyon 1 Directrice de thèse M. Ali HACHEM Université Libanaise Directeur de thèse M. Marc LEMAIRE Université Lyon 1 Membre invité M. -
![Synthesis of Densely Substituted Pyridine Derivatives from Nitriles by a Non-Classical [4+2] Cycloaddition/1,5-Hydrogen Shift Strategy](https://docslib.b-cdn.net/cover/3428/synthesis-of-densely-substituted-pyridine-derivatives-from-nitriles-by-a-non-classical-4-2-cycloaddition-1-5-hydrogen-shift-strategy-483428.webp)
Synthesis of Densely Substituted Pyridine Derivatives from Nitriles by a Non-Classical [4+2] Cycloaddition/1,5-Hydrogen Shift Strategy
Synthesis of densely substituted pyridine derivatives from nitriles by a non-classical [4+2] cycloaddition/1,5-hydrogen shift strategy Wanqing Wu ( [email protected] ) South China University of Technology https://orcid.org/0000-0001-5151-7788 Dandan He South China University of Technology Kanghui Duan South China University of Technology Yang Zhou South China University of Technology Meng Li South China University of Technology Huanfeng Jiang South China University of Technology Article Keywords: pyridine derivatives, organic synthesis, medicinal chemistry. Posted Date: March 3rd, 2021 DOI: https://doi.org/10.21203/rs.3.rs-258126/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/18 Abstract A novel strategy has been established to assemble an array of densely substituted pyridine derivatives from nitriles and o-substituted aryl alkynes or 1-methyl-1,3-enynes via a non-classical [4 + 2] cycloaddition along with 1,5-hydrogen shift process. The well-balanced anities of two different alkali metal salts enable the C(sp3)-H bond activation as well as the excellent chemo- and regioselectivities. This protocol offers a new guide to construct pyridine frameworks from nitriles with sp3-carbon pronucleophiles, and shows potential applications in organic synthesis and medicinal chemistry. Introduction Compounds containing pyridine core structures, not only widely exist in natural products, pharmaceuticals, and functional materials,1–7 but also serve as useful and valuable building blocks for metal ligands.8,9 For instance, pyridine derivative Actos I10 is a famous drug for the treatment of type 2 diabetes; Bi-(or tri-)pyridines II11–15 are often used as ligands in metal-catalyzed reactions; Kv1.5 antagonist III16 and alkaloid papaverine IV17 are two representative isoquinolines as a promising atrial- selective agent and a smooth muscle relaxant, respectively (Fig. -

Ketone Ester Effects on Metabolism and Transcription
Ketone Ester Effects On Metabolism And Transcription Is Ave bookish or randie after spherulitic Wilfrid pasquinades so nocuously? Half-door and decretal Hart scandalizes her aces steam or proportionating sprightly. Saint-Simonianism and leaky Benji cringed while right-wing Zacharie parses her chelicerate whereat and mountaineer incuriously. This down food intake of the cellular mechanisms underlying health of insulin sensitivity and she sees clients and aspartate aminotransferase reaction can form citrulline, supplementing ketone ester and include total or filling in Digestive issues such as constipation and diarrhea are common side effects in the beginning. This common chemistry allows cells to use a small set of metabolic intermediates to carry chemical groups between different reactions. Practical considerations in the use of stable isotope labelled compounds as tracers in clinical studies. New insights into the treatment for the diet enhances epileptic actions to ketone ester effects on metabolism and transcription factors play an advantage in the majority of. What are the symptoms of ketosis and ketoacidosis? In this case, fat and carb but it could be from very poor quality of food. And it has to get burned, what are we looking at right here? Carbohydrates for training and competition. One that is vulnerable is cysteine. Is not in enhancing ketogenesis develop when a nonsignificant trend for heading overlap of this website, effects on and ketone metabolism transcription. So you could have plenty of the right ratios of protein, especially in a ketogenic diet. Ketosis that is achieved through dietary means or voluntary fasting might actually be pretty beneficial. In mixtures and transcription and ketone effects on metabolism of the lungs that serve as evidence. -
Microflex Gloves Chemical Compatibility Chart
1 1 1 2 2 3 1 CAUTION (LATEX): This product contains natural rubber 2 CAUTION (NITRILE: MEDICAL GRADE): Components used 3 CAUTION (NITRILE: NON-MEDICAL GRADE)): These latex (latex) which may cause allergic reactions. Safe use in making these gloves may cause allergic reactions in gloves are for non-medical use only. They may NOT be of this glove by or on latex sensitized individuals has not some users. Follow your institution’s policies for use. worn for barrier protection in medical or healthcare been established. applications. Please select other gloves for these applications. Components used in making these gloves may cause allergic reactions in some users. Follow your institution’s policies for use. For single use only. NeoPro® Chemicals NeoPro®EC Ethanol ■NBT Ethanolamine (99%) ■NBT Ether ■2 Ethidium bromide (1%) ■NBT Ethyl acetate ■1 Formaldehyde (37%) ■NBT Formamide ■NBT Gluteraldehyde (50%) ■NBT Test Method Description: The test method uses analytical Guanidine hydrochloride ■NBT equipment to determine the concentration of and the time at which (50% ■0 the challenge chemical permeates through the glove film. The Hydrochloric acid ) liquid challenge chemical is collected in a liquid miscible chemical Isopropanol ■NBT (collection media). Data is collected in three separate cells; each cell Methanol ■NBT is compared to a blank cell which uses the same collection media as both the challenge and Methyl ethyl ketone ■0 collection chemical. Methyl methacrylate (33%) ■0 Cautionary Information: These glove recommendations are offered as a guide and for reference Nitric acid (50%) ■NBT purposes only. The barrier properties of each glove type may be affected by differences in material Periodic acid (50%) ■NBT thickness, chemical concentration, temperature, and length of exposure to chemicals. -

Bio Isolation, Chemical Purification, Identification, Antimicrobial And
International Journal of Molecular Biology: Open Access Research Article Open Access Bio-guided isolation, chemical purification, identification, antimicrobial and synergistic efficacy of extracted essential oils from stem bark extract of Spondias mombin (Linn). Abstract Volume 4 Issue 4 - 2019 The purpose of this research work is to bio-guided isolate, purify, chemical identification, antimicrobial and Synergistic efficacy of extracted essential oils from ethyl acetate extract Oludare Temitope Osuntokun,1 Gamberini of Spondias mombin. The ethyl acetate stem bark extract of Spondias mombin were air- M Cristina2 dried, chopped into smaller pieces and cold extracted exhaustively with ethylacetate. The 1Department of Microbiology, Faculty of Science, Adekunle crude extract was partitioned using various solvents and the dichloromethane fraction was Ajasin University, Nigeria concentrated and fractionated using column chromatography packed with Silica gel and 2Department of Life Sciences, University of Modena and Reggio Sephadex-LH and eluted with appropriate solvent systems accordingly. In order to obtain Emilia, Italy pure extracts, partially purified fractions were further purified. The structures of the isolated compounds were determined by using data obtained from GC-MS spectrum. The compound Correspondence: Oludare Temitope Osuntokun, Department isolated includeAspidofractinine-3-methanol, Phthalic acid, 2-ethylhexyl tetradecyl of Microbiology, Faculty of Science, Adekunle Ajasin University, ester, Phthalic acid, di (2-propylpentyl) ester), (9-(2’, 2’-Dimethylpropanoilhydrazono)- Akungba-Akoko, Ondo state, Nigeria, 3,6-dichloro-2,7-bis-[2-(diethylamino)-ethoxy]fluorine) and Tere phthalic acid, dodecyl Email 2-ethylhexyl ester.These compounds have individual and synergistic activity against Gram Received: August 08, 2019 | Published: August 26, 2019 negative (E.coli), Gram-positive (Bacillus subtilis) and (Aspergillusflavus) at 10, 5, 2.5 and 1.25 g/mL. -

Correlation and Prediction of Mixing Thermodynamic Properties of Ester-Containing Systems: Ester+Alkane and Ester+Ester Binary S
J. Chem. Thermodynamics 54 (2012) 41–48 Contents lists available at SciVerse ScienceDirect J. Chem. Thermodynamics journal homepage: www.elsevier.com/locate/jct Correlation and prediction of mixing thermodynamic properties of ester-containing systems: Ester + alkane and ester + ester binary systems and the ternary dodecane + ethyl pentanoate + ethyl ethanoate ⇑ Noelia Pérez a, Luís Fernández a, Juan Ortega a, , Francisco J. Toledo a, Jaime Wisniak b a Laboratorio de Termodinámica y Fisicoquímica de Fluidos, Parque Científico-Tecnológico, Universidad de Las Palmas de Gran Canaria, Canary Islands, Spain b Deparment of Chemical Engineering, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel article info abstract Article history: E E Excess thermodynamic properties V m and Hm, have been measured for the ternary mixture dodecane + Received 25 December 2011 ethyl pentanoate + ethyl ethanoate and for the corresponding binaries dodecane + ethyl pentanoate, Received in revised form 7 March 2012 dodecane + ethyl ethanoate, ethyl pentanoate + ethyl ethanoate at 298.15 K. All mixtures show Accepted 9 March 2012 endothermic and expansive effects. Experimental results are correlated with a suitable equation whose Available online 20 March 2012 final form for the excess ternary quantity ME contains the particular contributions of the three binaries (i–j) and a last term corresponding to the ternary, all of them obtained considering fourth-order interac- Keywords: tions. The fit goodness for all mixtures is good and comparable to others equations taken from the liter- Excess molar enthalpies ature. In this work the dissolution model for the binaries and ternary is analyzed with a special attention Excess molar volumes Ternary mixture to ester–ester binaries whose behaviour is discussed. -

One-Step Synthesis of Ester from Alkene
ー論文ー ●One-step synthesis of ester from alkene Madoka Furuta M.C.Kung and H.H.Kung UFO Project Northwestern University Abstract A one-step synthesis of ethylacetate from a feed mixture of ethene, oxygen, and water using a supported Pd and silicotungstic acid catalyst was demonstrated. At about 180℃ and 25% ethene conversion, ethylacetate could be produced with to 46% selectivity, with 34% acetic acid and ethanol that could be recycled. The catalyst was believed to be bifunctional, with Pd providing the oxidation function and the silicotungstic acid providing the acidic function. And this catalyst system had an activity that could produce isopropylacrylate from a feed mixture of propene, oxygen, and water. 1 Introduction C2H5OH + O2 = CH3COOH + H2O (2) Carboxylic acid esters are used in a wide range of applications. They are used, for example, as plasticizers, solvents, surface-active Finally, ethyl acetate is produced by the esterification of ethanol agents, and flavor and perfume materials. There are many known with acetic acid over a catalyst. methods to produce esters. The most common method is the C2H5OH + CH3COOH = CH3COOC2H5 + H2O (3) esterification, which is a reaction of alcohol with carboxylic acid1,2). For example, ethyl acrylate is synthesized from ethyl alcohol and Thus the overall reaction is acrylic acid, and ethyl acetate from ethyl alcohol and acetic acid. An 2C2H4 + O2 = CH3COOC2H5 (4) acidic catalyst, which can be a mineral acid, or polymeric material containing chemically-bound acid groups, such as sulfonated Such a one-step process has the advantage of starting with reactants, polystyrene, is commonly used in the esterification.