Acs-36-326.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Presbyterian Church in Taiwan and the Advocacy of Local Autonomy
SINO-PLATONIC PAPERS Number 92 January, 1999 The Presbyterian Church in Taiwan and the Advocacy of Local Autonomy by Christine Louise Lin Victor H. Mair, Editor Sino-Platonic Papers Department of East Asian Languages and Civilizations University of Pennsylvania Philadelphia, PA 19104-6305 USA [email protected] www.sino-platonic.org SINO-PLATONIC PAPERS is an occasional series edited by Victor H. Mair. The purpose of the series is to make available to specialists and the interested public the results of research that, because of its unconventional or controversial nature, might otherwise go unpublished. The editor actively encourages younger, not yet well established, scholars and independent authors to submit manuscripts for consideration. Contributions in any of the major scholarly languages of the world, including Romanized Modern Standard Mandarin (MSM) and Japanese, are acceptable. In special circumstances, papers written in one of the Sinitic topolects (fangyan) may be considered for publication. Although the chief focus of Sino-Platonic Papers is on the intercultural relations of China with other peoples, challenging and creative studies on a wide variety of philological subjects will be entertained. This series is not the place for safe, sober, and stodgy presentations. Sino-Platonic Papers prefers lively work that, while taking reasonable risks to advance the field, capitalizes on brilliant new insights into the development of civilization. The only style-sheet we honor is that of consistency. Where possible, we prefer the usages of the Journal of Asian Studies. Sinographs (hanzi, also called tetragraphs [fangkuaizi]) and other unusual symbols should be kept to an absolute minimum. Sino-Platonic Papers emphasizes substance over form. -

Serine Protease Inhibitor SERPINE2 Reversibly Modulates Murine Sperm Capacitation
International Journal of Molecular Sciences Article Serine Protease Inhibitor SERPINE2 Reversibly Modulates Murine Sperm Capacitation Sheng-Hsiang Li 1,2, Yuh-Ming Hwu 1,2,3,4, Chung-Hao Lu 3, Ming-Huei Lin 2,3, Ling-Yu Yeh 1 and Robert Kuo-Kuang Lee 1,3,5,* 1 Department of Medical Research, Mackay Memorial Hospital, Tamsui District, New Taipei City 251, Taiwan; [email protected] (S.-H.L.); [email protected] (Y.-M.H.); [email protected] (L.-Y.Y.) 2 Mackay Junior College of Medicine, Nursing, and Management, Beitou District, Taipei City 112, Taiwan; [email protected] 3 Department of Obstetrics and Gynecology, Mackay Memorial Hospital, Taipei City 104, Taiwan; [email protected] 4 Mackay Medical College, Sanzhi District, New Taipei City 252, Taiwan 5 Department of Obstetrics and Gynecology, Taipei Medical University, Taipei City 110, Taiwan * Correspondence: [email protected]; Tel.: +886-2-2543-3535 Received: 2 May 2018; Accepted: 17 May 2018; Published: 19 May 2018 Abstract: SERPINE2 (serpin peptidase inhibitor, clade E, member 2), predominantly expressed in the seminal vesicle, can inhibit murine sperm capacitation, suggesting its role as a sperm decapacitation factor (DF). A characteristic of DF is its ability to reverse the capacitation process. Here, we investigated whether SERPINE2 can reversibly modulate sperm capacitation. Immunocytochemical staining revealed that SERPINE2 was bound onto both capacitated and uncapacitated sperm. It reversed the increase in BSA-induced sperm protein tyrosine phosphorylation levels. The effective dose and incubation time were found to be >0.1 mg/mL and >60 min, respectively. Calcium ion levels in the capacitated sperm were reduced to a level similar to that in uncapacitated sperm after 90 min of incubation with SERPINE2. -

The Three-In-One Assistive Technologies for Seniors—Support, Move, Table
Technical Report Page 1 of 7 The three-in-one assistive technologies for seniors—support, move, table Hsiu-Yang Hsu1, Jung-Mei Tsai1,2, Miao Wu1 1Department of Nursing, Mackay Memorial Hospital, New Taipei City 25160, Taiwan; 2Department of Nursing, Mackay Medical College, Mackay Junior College of Medicine, Nursing, and Management and College of Nursing and Health Sciences, DAYEH University, Taiwan Correspondence to: Miao Wu. Department of Nursing, Mackay Memorial Hospital, No.45, Min-sheng Rd., Tamsui District, New Taipei City 25160, Taiwan. Email: [email protected]. Abstract: We offer the “supportive = assistive” technologies senior citizens need in order to improve the safety of medical visiting, the quality of medical caring, and the satisfaction with environmental security. The Three-in-One Assistive Technologies for Seniors are designed in accordance with the present circumstance to not only be available as clinic equipment and facilities, but also extensive for the continual use of wards or households. In this regard, the goal of this innovative design is to make them—medical apparatus, personal movement assistive technologies, daily living assistive technologies—equivalent. Keywords: Assistive technologies; innovative design; senior care; moving safety Received: 28 August 2018; Accepted: 21 May 2019; published: 28 June 2019. doi: 10.21037/ht.2019.06.01 View this article at: http://dx.doi.org/10.21037/ht.2019.06.01 Motivation for invention The rate of Taiwanese old age population (aged over 65) reached 7 percent in 1993 and went up to 12.51 percent at the end of 2015 (Department of Statistics, Ministry of the Interior, 2016). To meet the needs of the aging population, safety measures should be properly added to medical equipment and environment, establishing a friendlier and safer medical environment for senior citizens. -

2020 WHA.Pdf
HEALTH FOR ALL TAIWAN CAN HELP Clarion Call he global outbreak of coronavirus, for many to better understand and fully appreci- fi rst reported in the Chinese city of ate the ways Taiwan Can Help. CLARION CALL 03 Wuhan late last year, is presenting Necessity is the mother of invention. Taiwan the international community with has taken the spirit of this proverb to heart, HEALTH NEWS 04 an unprecedented array of economic, medical, using it to shape a policy applied with great vim political and social challenges. Taiwan, on the and vigor to coronavirus-fighting efforts. When 08 T LEADING BY EXAMPLE front lines of the COVID-19 pandemic less signs first surfaced of atypical pneumonia cases INTERVIEW BY OSCAR CHUNG than 130 kilometers from China, is not immune. in Wuhan, the Ministry of Health and Welfare In fact, the country is soldiering on outside (MOHW) and Centers for Disease Control 12 EXPANDING OUTREACH the support network of the World Health (CDC) drew on the country’s deep experience Reprinted from January / February 2020 issue of Taiwan Review BY KELLY HER Organization (WHO). with severe acute respiratory syndrome and This wholly unsatisfactory state of affairs immediately took action. NEW LEASE ON LIFE 18 is the direct result of China’s refusal to allow This included carrying out onboard health BY MEG CHANG Taiwan a voice in the WHO. Despite a highly inspections of passengers returning from Wuhan, touted National Health Insurance system and fever screening of arrivals, and contact, occupation BORDERLESS CARE 20 a long track record of successful cross-border and travel assessments. -

COVID-19 in Older Adults: a Focus on Clinical Characteristics and Frail Status
International Journal of Gerontology 14 (2020) 256-259 https://doi.org/10.6890/IJGE.202011_14(4).0001 International Journal of Gerontology journal homepage: http://www.sgecm.org.tw/ijge/ Review Article COVID-19 in Older Adults: A Focus on Clinical Characteristics and Frail Status Chih-Po Chang a#, Ching-Hui You d#, Ling-Yi Tseng f,g, Yun-Ju Cheng a,g,h, Hsiang-Kuang Tseng a,b,c,e * a Division of Geriatric Medicine, Mackay Memorial Hospital, Taipei, Taiwan, b Division of Infectious Disease, Mackay Memorial Hospital, Taipei, Taiwan, c Department of Internal Medicine, Mackay Memorial Hospital, Taipei, Taiwan, d Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, United States of America, e Department of Medicine, Institute of Long-term Care, MacKay Medical College, New Taipei City, Taiwan, f Department of English, National Taiwan Normal University, Taipei, Taiwan, g Department of Health Promotion and Health Education, National Taiwan Normal University, Taipei, Taiwan; h Department of Health Care and Social Work, Taipei University of Marine Technology, New Taipei City, Taiwan ARTICLE INFO SUMMARY Accepted 13 August 2020 Worldwide attention has been drawn to the recent COVID-19 outbreak. Many studies have shown that under the pandemic, elderlies, especially those with chronic diseases, are more vulnerable than youths. Keywords: Upon infection, older people tend to endure a higher hospitalization and mortality rate, and the mor- COVID-19, tality rate after acute hospitalization appears even higher for frail elderlies than non-frail ones. More- old adults, over, older COVID-19 patients can exhibit different, atypical clinical manifestations such as falls, de- clinical characteristics, lirium, general weakness, functional decline, and other geriatric syndromes indicating frailty. -

CALR Mutations in Myeloproliferative Neoplasms*
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector International Journal of Gerontology 8 (2014) 105 Contents lists available at ScienceDirect International Journal of Gerontology journal homepage: www.ijge-online.com Letter to the Editor CALR Mutations in Myeloproliferative Neoplasms* * To the Editor, Ken-Hong Lim Division of Hematology and Oncology, Department of Internal Medicine, The discovery of the JAK2V617F mutation has provided impor- Mackay Memorial Hospital, Taiwan tant insight into the pathogenesis of BCR-ABL1-negative myelopro- Laboratory of Good Clinical Research Center, Department of Medical Research, liferative neoplasms (MPNs) and has revolutionized the diagnosis Mackay Memorial Hospital, Tamsui District, Taiwan and treatment of MPNs. Following JAK2V617F mutation, somatic mutations such as JAK2 exon 12, MPL, LNK, TET2, DNMT3A, IDH1/2, Graduate Institute of Oncology, National Taiwan University College of Medicine, ASXL1, EZH2, SRSF2, and SF3B1 have been identified in MPNs. We Taipei, Taiwan have recently reported the frequency of JAK2V617F and DNMT3A Mackay Medical College, New Taipei City, Taiwan mutations in adult Taiwanese patients with BCR-ABL1-negative Huan-Chau Lin MPNs, and the results are comparable with those in Western coun- Division of Hematology and Oncology, Department of Internal Medicine, 1,2 tries . However, JAK2V617F, MPL, and DNMT3A mutations were not Mackay Memorial Hospital, Taiwan detected in approximately 40% of patients with essential thrombo- cythemia (ET) in our cohort and others’ cohorts. With the help of Laboratory of Good Clinical Research Center, Department of Medical Research, the next-generation sequencing technique, two study groups Mackay Memorial Hospital, Tamsui District, Taiwan have recently demonstrated a high frequency of calreticulin Caleb Gon-Shen Chen (CALR) exon 9 mutations in patients with JAK2V617F-negative ET Division of Hematology and Oncology, Department of Internal Medicine, fi 3,4 and primary myelo brosis . -
臺勢教會 the Taiwanese Making of the Canada Presbyterian Mission
臺勢教會 The Taiwanese Making of the Canada Presbyterian Mission Mark A. Dodge Series in World History Copyright © 2021 by the author. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of Vernon Art and Science Inc. www.vernonpress.com In the Americas: In the rest of the world: Vernon Press Vernon Press 1000 N West Street, Suite 1200 C/Sancti Espiritu 17, Wilmington, Delaware, 19801 Malaga, 29006 United States Spain Series in World History Library of Congress Control Number: 2020947486 ISBN: 978-1-64889-119-9 Cover design by Vernon Press. Cover image: George Leslie Mackay, native pastors, and students during itinerating in North Formosa. Aletheia University Archives AUP0000111. Product and company names mentioned in this work are the trademarks of their respective owners. While every care has been taken in preparing this work, neither the authors nor Vernon Art and Science Inc. may be held responsible for any loss or damage caused or alleged to be caused directly or indirectly by the information contained in it. Every effort has been made to trace all copyright holders, but if any have been inadvertently overlooked the publisher will be pleased to include any necessary credits in any subsequent reprint or edition. Table of contents List of Figures v Acknowledgements vii A Note on the Romanization of Chinese ix Introduction: The Miracle Mission xiii Chapter 1 -

Cryptococcemia in an Elderly Woman with Retroperitoneal Diffuse Large B-Cell Lymphoma After Rituximab-Containing Chemotherapy*
International Journal of Gerontology 10 (2016) 112e116 Contents lists available at ScienceDirect International Journal of Gerontology journal homepage: www.ijge-online.com Case Report Cryptococcemia in an Elderly Woman with Retroperitoneal Diffuse Large B-cell Lymphoma after Rituximab-containing Chemotherapy* Ming-Wei Cheng 1, Alice Ying-Jung Wu 1, Chang-Pang Liu 1, 2, 3, Ken-Hong Lim 2, 4, * Shu-Ling Weng 5, Hsiang-Kuang Tseng 1, 2, 3 1 Division of Infectious Diseases, Department of Internal Medicine, MacKay Memorial Hospital, Zhongshan District, Taipei City, 2 School of Medicine, MacKay Medical College, New Taipei, 3 Microbiology Section, Department of Medical Research, MacKay Memorial Hospital, 4 Division of Hematology and Oncology, Department of Internal Medicine, MacKay Memorial Hospital, 5 Department of Laboratory Medicine, MacKay Memorial Hospital, Taipei, Taiwan article info summary Article history: Cryptococcemia is a bloodstream fungal infection caused by encapsulated yeasts of Cryptococcus neo- Received 20 January 2015 formans. We reported an 88-year-old woman in whom C. neoformans colonies of smooth and mucoid Received in revised form phenotypes were sequentially cultured from the blood during treatment of retroperitoneal diffuse large 24 February 2015 B-cell lymphoma using rituximab-containing chemotherapy. The most likely cause was disease pro- Accepted 25 February 2015 gression of pulmonary cryptococcoma. She was successfully treated with optimal antifungal therapy. Available online 14 June 2016 Copyright © 2016, Taiwan Society of Geriatric Emergency & Critical Care Medicine. Published by Elsevier Taiwan LLC. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/ Keywords: cryptococcemia, licenses/by-nc-nd/4.0/). -

Schsu 95259 PST90 Info.Pdf
Sigma's 30th International Nursing Research Congress Symptom Distress, Illness Intrusiveness, Hope, and Depression in Dialysis Patients Shu-Ching Hsu, MS, RN, ARNP School of Nursing, National Taipei University of Nursing and Health Sciences/ MacKay Memorial Hospital, Taipei, Taiwan Tsae-Jyy Wang, PhD, RN, ARNP School of Nursing, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan InFun Li, PhD, RN Department of Nursing, Tamsui MacKay Memorial Hospital, New Taipei City, Taiwan Purpose: The prevalence of depression is 33.33% to 78.8% for hemodialysis patients, 19% to 51.5% for peritoneal dialysis patients, and 20% to 45% for kidney transplant recipients. Depression can increase symptoms, reduce treatment compliance, and thus increase hospitalization and morbidity rate. So we exploring the Influence of Biochemistry, Disease characteristics, Symptom Distress, Illness Intrusiveness, Social Support, and Hope on Depression in Dialysis Patients. Understanding the factors that influence depression can help to screen for high-risk groups and develop preventive measures for depression. Methods: The purpose of this study was to investigate depression among dialysis patients and its influencing factors, including demographics, disease characteristics, serum values (calcium, phosphorus, hemoglobin), symptom distress, illness intrusiveness, social support, and a sense of hope. The study used a descriptive study design. A convenience sample of 130 dialysis patients with end-stage renal diseases were recruited from a medical center in northern Taiwan. The data were collected by using a structured questionnaire, including questions on demographics, the Symptom Distress Scale, the Illness Intrusiveness Ratings Scale, the Social Support Scale, the Herth Hope Index and the Center for Epidemiological Studies Depression Scale. -
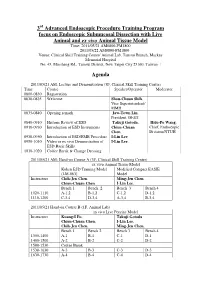
3 Advanced Endoscopic Procedure Training Program Focus On
3rd Advanced Endoscopic Procedure Training Program focus on Endoscopic Submucosal Dissection with Live Animal and ex vivo Animal Tissue Model Time: 2011/05/21 AM0800-PM1800 2011/05/22 AM0800-PM1800 Venue: Clinical Skill Training Center/ Animal Lab, Tamsui Branch, Mackay Memorial Hospital No. 45, Minsheng Rd., Tamsui District, New Taipei City 25160, Taiwan Agenda 2011/05/21 AM: Lecture and Demonstration (3F, Clinical Skill Training Center) Time Course Speaker/Operator Moderator 0800-0830 Registration 0830-0835 Welcome Shou-Chuan Shih , Vice Superintendent/ MMH 0835-0840 Opening remark Jaw-Town Lin , President, DEST, 0840-0910 Historic Review of ESD Takuji Gotoda , Hsiu-Po Wang , 0910-0930 Introduction of ESD Instruments Chien-Chuan Chief, Endoscopic Chen , Division/NTUH 0930-0950 Introduction of ESD/EMR Procedure I-Lin Lee , 0950-1010 Video or ex vivo Demonstration of I-Lin Lee , ESD Basic Skills 1010-1020 Coffee Break & Change Dressing 2011/05/21 AM: Hand-on Course A (3F, Clinical Skill Training Center) ex vivo Animal Tissue Model Koken ESD Training Model Modified Compact EASIE (LM-083) Model Instructors Chih-Jen Chen Ming-Jen Chen , Chien-Chuan Chen I-Lin Lee , Bench 1 Bench 2 Bench 3 Bench 4 1020-1110 A-1,2 B-1,2 C-1,2 D-1,2 1110-1200 C-3,4 D-3,4 A-3,4 B-3,4 2011/05/21 Hand-on Course B (1F, Animal Lab) in vivo Live Porcine Model Instructors Kuang-I Fu , Takuji Gotoda Chien-Chuan Chen , I-Lin Lee , Chih-Jen Chen , Ming-Jen Chen , Bench 1 Bench 2 Bench 3 Bench 4 1300-1400 A-1 B-1 C-1 D-1 1400-1500 A-2 B-2 C-2 D-2 1500-1530 Coffee Break -

November 2020 | Vol
Issue Sponsor 號 執 照 登 記 為 雜 誌 交 寄 THE AMERICAN CHAMBER OF COMMERCE IN TAIPEI TAIWAN BUSINESS TOPICS November 2020 | Vol. 50 | Issue 11 中 華 郵 政 北 台 字 第 5000 透過將生命放在第一位, 我們開創了永久的傳承 在過去近 130 年來,我們致力為世界各地人類及動物所面對最棘 手的健康挑戰研發對抗疾病的希望,今日,默沙東持續成為全球 一流的研發密集型生物製藥公司,為今日、明日的病患及社會, 甚或是未來的世代不斷帶來更多的醫學創新。 Copyright © 2020 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. All rights reserved. TW-NON-00261 美商默沙東藥廠股份有限公司台灣分公司 地址:台北市信義路五段 106 號 12 樓 電話 : (02)6631-6000 CONTENTS NEWS AND VIEWS 7 Editorial NOVEMBER 2020 VOLUME 50, NUMBER 11 Reexamining Strategic Ambiguity 一○九年十一月號 重新檢視戰略模糊 8 Taiwan Briefs Publisher 發行人 By Jeremy Olivier Leo Seewald 李豪 Editor-in-Chief 總編輯 14 Issues Don Shapiro 沙蕩 Is Taiwan Ready for a Sovereign Deputy Editor 副總編輯 Jeremy Olivier 歐嘉仁 6 President’s View Wealth Fund? Art Director/ 美術主任/ 主權基金行不行? Out with the old and in with the new Production Coordinator 後製統籌 Katia Chen 陳國梅 By Leo Seewald By Don Shapiro Manager, Publications Sales & Marketing 廣告行銷經理 Caroline Lee 李佳紋 COVER SECTION Translation 翻譯 Kevin Chen, Andrew Wang 台灣醫療體系總體檢 陳又銘, 王先棠 Checking Up on Taiwan’s Hospitals American Chamber of Commerce in Taipei 17 Controlling Costs While 129 MinSheng East Road, Section 3, 7F, Suite 706, Taipei 10596, Taiwan Fighting Disease P.O. Box 17-277, Taipei, 10419 Taiwan Tel: 2718-8226 Fax: 2718-8182 Aside from the large medical e-mail: [email protected] website: http://www.amcham.com.tw centers that enjoy significant 名稱:台北市美國商會工商雜誌 economies of scale, many 發行所:台北市美國商會 臺北市10596民生東路三段129號七樓706室 Taiwanese hospitals have 電話:2718-8226 傳真:2718-8182 been faced with financial Taiwan Business Topics is a publication of the American Chamber of Commerce in Taipei, ROC. -

The Legacy of George Leslie Mackay James R
The Legacy of George Leslie Mackay James R. Rohrer eorge Leslie Mackay (1844–1901) was among the most of his death. Presbyterian youth groups can be spotted wearing Gremarkable missionaries of the late Victorian era. Dur- T-shirts bearing Mackay’s likeness, along with his motto, “It is ing his three decades in Taiwan (1871–1901), he received little better to burn up than rust out.” Christian parents can read chil- substantive assistance from other Canadian missionaries yet, at dren to sleep with tales of Mackay printed in cartoon storybooks, the time of his death, left a community of more than 2,400 bap- while a seemingly endless stream of newspaper articles, art prints, tized communicants and a much larger body of regular hearers postcards, posters, wall calendars, mugs, and medallions recalls who attended more than sixty churches led by full-time native his life. Preachers in Taiwan frequently draw upon his book, From preachers.1 Mackay (pronounced “muh-KIGH”) established a Far Formosa (1895), for sermon illustrations, and politicians have hospital, a school for girls, and “Oxford College,” his training appealed to his memory to promote various agendas. center for native church leaders. Just as important, as one of only Indeed, the legendary Mackay at times threatens to eclipse three known missionaries in nineteenth-century China to have the historical missionary. As the Canadian who became “the married an indigenous spouse, he left behind descendents who son-in-law of Taiwan,” Mackay has served as a useful icon for continued to play leading roles in Taiwan’s Presbyterian Church Taiwanese nationalism, a symbol of the warm ties binding the after his death.