Download PDF File
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparison of the Newborn Skull to the Adult Human Skull
Comparison of the Newborn skull to the Adult Human Skull As a baby grows older their skull goes through a huge change. The neurocranium starts off not as hard as it will be, gaining the ability to shape in whatever way is needed. While their facial cranium, their face, begins to take on unique qualities and changes to look like a mature adult skull. This process takes time but all the changes are very visible. The neurocranium compared to an adult’s is more oval and is substantially bigger than the facial cranium. The newborn's skull has four “horns” two in the front on the frontal bone and two in the back on the parietal bone. These bumps are the thickness that the skull will eventually become. The edges are ridged in between the frontal and the parietal. On top of the skull is the anterior fontanel, which is an opening in the skull that is small and shaped like a diamond. This will close when the child is around two years old. Coming out of the points from the anterior fontanelle are lines or spaces in between the bones, some of these overlap. The advantage of both the spaces in between the bones and the anterior fontanel is room for growth and compression through the birth canal. As a newborn, their neurocranium is 60% of the circumference of an adult’s. At two to three it is 90% of an adult’s, so most of the growth of the neurocranium happens before the child is three. The adult’s skull is more circular and the nose, eyes, and mouth are father apart. -

Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Morphology of the Pterion in Serbian Population
Int. J. Morphol., 38(4):820-824, 2020. Morphology of the Pterion in Serbian Population Morfología del Pterion en Población Serbia Knezi Nikola1; Stojsic Dzunja Ljubica1; Adjic Ivan2; Maric Dusica1 & Pupovac Nikolina4 KNEZI, N.; STOJSIC, D. L.; ADJIC, I.; MARIC, D. & PUPOVAC, N. Morphology of the pterion in Serbian population. Int. J. Morphol., 38(4):820-824, 2020. SUMMARY: The pterion is a topographic point on the lateral aspect of the skull where frontal, sphenoid, parietal and temporal bones form the H or K shaped suture. This is an important surgical point for the lesions in anterior and middle cranial fossa. This study was performed on 50 dry skulls from Serbian adult individuals from Department of Anatomy, Faculty of Medicine in Novi Sad. The type of the pterion on both sides of each skull was determined and they are calcified in four types (sphenoparietal, frontotemporal, stellate and epipteric). The distance between the center of the pterion and defined anthropological landmarks were measured using the ImageJ software. Sphenoparietal type is predominant with 86 % in right side and 88 % in left side. In male skulls, the distance from the right pterion to the frontozygomatic suture is 39.89±3.85 mm and 39.67±4.61 mm from the left pterion to the frontozygomatic suture. In female skulls the distance is 37.38±6.38 mm on the right and 35.94±6.46 mm on the left. The shape and the localization of the pterion are important because it is an anatomical landmark and should be used in neurosurgery, traumatology and ophthalmology. -

CLOSURE of CRANIAL ARTICULATIONS in the SKULI1 of the AUSTRALIAN ABORIGINE by A
CLOSURE OF CRANIAL ARTICULATIONS IN THE SKULI1 OF THE AUSTRALIAN ABORIGINE By A. A. ABBIE, Department of Anatomy, University of Adelaide INTRODUCTION While it is well known that joint closure advances more or less progressively with age, there is still little certainty in matters of detail, mainly for lack of adequate series of documented skulls. In consequence, sundry beliefs have arisen which tend to confuse the issue. One view, now disposed of (see Martin, 1928), is that early suture closure indicates a lower or more primitive type of brain. A corollary, due to Broca (see Topinard, 1890), that the more the brain is exercised the more is suture closure postponed, is equally untenable. A very widespread belief is based on Gratiolet's statement (see Topinard, 1890; Frederic, 1906; Martin, 1928; Fenner, 1939; and others) that in 'lower' skulls the sutures are simple and commence to fuse from in front, while in 'higher' skulls the sutures are more complicated and tend to fuse from behind. This view was disproved by Ribbe (quoted from Frederic, 1906), who substituted the generalization that in dolicocephals synostosis begins in the coronal suture, and in brachycephals in the lambdoid suture. In addition to its purely anthropological interest the subject raises important biological considerations of brain-skull relationship, different foetalization in different ethnological groups (see Bolk, 1926; Weidenreich, 1941; Abbie, 1947), and so on. A survey of the literature reveals very little in the way of data on the age incidence of suture closure. The only substantial contribution accessible here comes from Todd & Lyon (1924) for Europeans, but their work is marred by arbitrary rejection of awkward material. -

Real-Time Sonographic Sector Scanning of the Neonatal Cranium: Technique and Normal Anatomy
349 Real-Time Sonographic Sector Scanning of the Neonatal Cranium: Technique and Normal Anatomy William P. Shuman 1 A commercially available wide field of view real-time mechanical sector scanner can James V. Rogers1 be used t o image the neonatal cranium. Because of the small transducer head size, the 1 Laurence A. Mack . 2 open anterior fontanelle can function as an acoustic window. By thus avoiding bone, higher f requency transducers may be used to improve image resolution. Infants may Ellsworth C. Alvord, Jr 3 be scanned quickly without sedation in their isolettes in the neonatal intensive care David P. Christie2 unit. Sterility of the infant environment is maintained by placing the transducer in a surgical glove. Using this technique, detailed normal anatomy can be seen such as vascular structures, caudate nucleus, thalamus, third ventricle, cavum septum pelluci dum, and the thalamocaudate notch. Angled coronal and sagittal sonographic anatomy is correlated with neonatal cadaver brain sections sliced in similar planes centered on the anterior fontanelle. The mechanical sector scanner has advantages over other real-time devices including improved image resolution, a wider field of view, and a small er area of transducer skin contact. These unique assets are particularly appli cable to th e evaluati on of the neonatal cranium. The small transducer of a sector scanner can easil y be held in contact with the open anterior fontanell e using it as an acousti c window. This window avoids bone and makes possible the use of a higher frequency transducer resulting in improved resolution. We report our technique for neonatal crani al sonography usin g a mechanical sector scanner. -
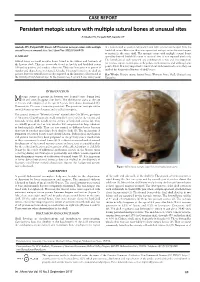
Persistent Metopic Suture with Multiple Sutural Bones at Unusual Sites
CASE REPORT Persistent metopic suture with multiple sutural bones at unusual sites Ambade HV, Fulpatil MP, Kasote AP Ambade HV, Fulpatil MP, Kasote AP. Persistent metopic suture with multiple in a human skull at asterion, left pterion and right coronal suture apart from the sutural bones at unusual sites. Int J Anat Var. 2017;10(3):69-70. lambdoid suture. Moreover, there was a persistent metopic suture between bregma to nasion in the same skull. The metopic suture with multiple sutural bones SUMMARY spreading beyond lambdoid suture at unusual sites is not reported previously. The knowledge of such variation and combination is rare and very important Sutural bones are small irregular bones found in the sutures and fontanels of for forensic expert, radiologists, orthopedists, neurosurgeons and anthropologist the human skull. They are commonly found at lambda and lambdoid suture point of view. It is very important to know about such variation because they can followed by pterion; and rarely at other sites. They vary from person to person in mislead the diagnosis of fracture of skull bones. number and shape, hence not named. Usually, 1-3 sutural bones in one skull are present, but 8-10 sutural bones are also reported in the literature, all restricted in Key Words: Metopic suture; Sutural bones; Wormian bones; Skull; Unusual sites; the vicinity of lambdoid sutures. In the present case, 8 sutural bones were present Variations INTRODUCTION etopic suture is present in between two frontal bones during fetal Mlife and soon disappear after birth. The obliteration starts at the age of 2 years and completed at the age of 8 years from above downwards (1). -

Spontaneous Encephaloceles of the Temporal Lobe
Neurosurg Focus 25 (6):E11, 2008 Spontaneous encephaloceles of the temporal lobe JOSHUA J. WIND , M.D., ANTHONY J. CAPUTY , M.D., AND FABIO ROBE R TI , M.D. Department of Neurological Surgery, George Washington University, Washington, DC Encephaloceles are pathological herniations of brain parenchyma through congenital or acquired osseus-dural defects of the skull base or cranial vault. Although encephaloceles are known as rare conditions, several surgical re- ports and clinical series focusing on spontaneous encephaloceles of the temporal lobe may be found in the otological, maxillofacial, radiological, and neurosurgical literature. A variety of symptoms such as occult or symptomatic CSF fistulas, recurrent meningitis, middle ear effusions or infections, conductive hearing loss, and medically intractable epilepsy have been described in patients harboring spontaneous encephaloceles of middle cranial fossa origin. Both open procedures and endoscopic techniques have been advocated for the treatment of such conditions. The authors discuss the pathogenesis, diagnostic assessment, and therapeutic management of spontaneous temporal lobe encepha- loceles. Although diagnosis and treatment may differ on a case-by-case basis, review of the available literature sug- gests that spontaneous encephaloceles of middle cranial fossa origin are a more common pathology than previously believed. In particular, spontaneous cases of posteroinferior encephaloceles involving the tegmen tympani and the middle ear have been very well described in the medical literature. -

MBB: Head & Neck Anatomy
MBB: Head & Neck Anatomy Skull Osteology • This is a comprehensive guide of all the skull features you must know by the practical exam. • Many of these structures will be presented multiple times during upcoming labs. • This PowerPoint Handout is the resource you will use during lab when you have access to skulls. Mind, Brain & Behavior 2021 Osteology of the Skull Slide Title Slide Number Slide Title Slide Number Ethmoid Slide 3 Paranasal Sinuses Slide 19 Vomer, Nasal Bone, and Inferior Turbinate (Concha) Slide4 Paranasal Sinus Imaging Slide 20 Lacrimal and Palatine Bones Slide 5 Paranasal Sinus Imaging (Sagittal Section) Slide 21 Zygomatic Bone Slide 6 Skull Sutures Slide 22 Frontal Bone Slide 7 Foramen RevieW Slide 23 Mandible Slide 8 Skull Subdivisions Slide 24 Maxilla Slide 9 Sphenoid Bone Slide 10 Skull Subdivisions: Viscerocranium Slide 25 Temporal Bone Slide 11 Skull Subdivisions: Neurocranium Slide 26 Temporal Bone (Continued) Slide 12 Cranial Base: Cranial Fossae Slide 27 Temporal Bone (Middle Ear Cavity and Facial Canal) Slide 13 Skull Development: Intramembranous vs Endochondral Slide 28 Occipital Bone Slide 14 Ossification Structures/Spaces Formed by More Than One Bone Slide 15 Intramembranous Ossification: Fontanelles Slide 29 Structures/Apertures Formed by More Than One Bone Slide 16 Intramembranous Ossification: Craniosynostosis Slide 30 Nasal Septum Slide 17 Endochondral Ossification Slide 31 Infratemporal Fossa & Pterygopalatine Fossa Slide 18 Achondroplasia and Skull Growth Slide 32 Ethmoid • Cribriform plate/foramina -

Cranial Suture Mesenchymal Stem Cells: Insights and Advances
biomolecules Review Cranial Suture Mesenchymal Stem Cells: Insights and Advances Bo Li 1, Yigan Wang 1, Yi Fan 2, Takehito Ouchi 3 , Zhihe Zhao 1,* and Longjiang Li 4,* 1 State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Department of Orthodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, China; [email protected] (B.L.); [email protected] (Y.W.) 2 State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Department of Cariology and Endodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, China; [email protected] 3 Department of Physiology, Tokyo Dental College, Tokyo 1010061, Japan; [email protected] 4 State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Department of Head and Neck Oncology, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, China * Correspondence: [email protected] (Z.Z.); [email protected] (L.L.) Abstract: The cranial bones constitute the protective structures of the skull, which surround and protect the brain. Due to the limited repair capacity, the reconstruction and regeneration of skull defects are considered as an unmet clinical need and challenge. Previously, it has been proposed that the periosteum and dura mater provide reparative progenitors for cranial bones homeostasis and injury repair. In addition, it has also been speculated that the cranial mesenchymal stem cells reside in the perivascular niche of the diploe, namely, the soft spongy cancellous bone between the interior and exterior layers of cortical bone of the skull, which resembles the skeletal stem cells’ distribution pattern of the long bone within the bone marrow. -

MORPHOMETRIC STUDY of PTERION in DRY ADULT HUMAN SKULLS Pratima Kulkarni 1, Shivaji Sukre 2, Mrunal Muley *3
International Journal of Anatomy and Research, Int J Anat Res 2017, Vol 5(3.3):4365-68. ISSN 2321-4287 Original Research Article DOI: https://dx.doi.org/10.16965/ijar.2017.337 MORPHOMETRIC STUDY OF PTERION IN DRY ADULT HUMAN SKULLS Pratima Kulkarni 1, Shivaji Sukre 2, Mrunal Muley *3. 1 Associate Professor, Department of Anatomy, G.M.C. Aurangabad, Maharashtra, India. 2 Professor and Head of department, Department of Anatomy, G.M.C. Aurangabad, Maharashtra, India. *3 Assistant Professor, Department of Anatomy, G.M.C. Aurangabad, Maharashtra, India. ABSTRACT Introduction: The pterion corresponds to the site of anterolateral fontanelle of the neonatal skull which closes at third month after birth. In the pterional fractures the anterior and middle meningeal arterial ramus ruptures commonly which results in extradural hemorrhage. Pterional approach is most suitable and minimally invasive approach in neurosurgery. Materials and Methods: The present study was carried out on the pterion of 36 dry adult skulls of known sex from department of anatomy GMC Aurangabad Maharashtra. Results: The mean and standard deviation of the distance between the centre of pterion to various anatomical landmarks. The distance between Pterion- frontozygomatic (P-FZ) suture 29.81±4.42mm on right side, 29.81±4.07mm on left side; Pterion-Zygomatic arch (P-Z) 37.16±3.77mm on right side, 37.56±3.71mm on left side, Pterion-asterion (P-A) 89.73±6.16mm on right side, 89.46±6.35mm on left side; Pterion-external acoustic meatus (P- EAM) 53.40±7.28mm on right side, 53.57±6.73mm on left side, Pterion- Mastoid process (P-M) 80.35±3.44mm on right side, 80.96±3.79mm on left side and Pterion- Pterion (P-P) 194.54±16.39mm were measured. -

Ectocranial Suture Closure in Pan Troglodytes and Gorilla Gorilla: Pattern and Phylogeny James Cray Jr.,1* Richard S
AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY 136:394–399 (2008) Ectocranial Suture Closure in Pan troglodytes and Gorilla gorilla: Pattern and Phylogeny James Cray Jr.,1* Richard S. Meindl,2 Chet C. Sherwood,3 and C. Owen Lovejoy2 1Department of Anthropology, University of Pittsburgh, Pittsburgh, PA 15260 2Department of Anthropology and Division of Biomedical Sciences, Kent State University, Kent, OH 44242 3Department of Anthropology, The George Washington University, Washington, DC 20052 KEY WORDS cranial suture; synostosis; variation; phylogeny; Guttman analysis ABSTRACT The order in which ectocranial sutures than either does with G. gorilla, we hypothesized that this undergo fusion displays species-specific variation among phylogenetic relationship would be reflected in the suture primates. However, the precise relationship between suture closure patterns of these three taxa. Results indicated that closure and phylogenetic affinities is poorly understood. In while all three species do share a similar lateral-anterior this study, we used Guttman Scaling to determine if the closure pattern, G. gorilla exhibits a unique vault pattern, modal progression of suture closure differs among Homo which, unlike humans and P. troglodyte s, follows a strong sapiens, Pan troglodytes,andGorilla gorilla.BecauseDNA posterior-to-anterior gradient. P. troglodytes is therefore sequence homologies strongly suggest that P. tr og lodytes more like Homo sapiens in suture synostosis. Am J Phys and Homo sapiens share a more recent common ancestor Anthropol 136:394–399, 2008. VC 2008 Wiley-Liss, Inc. The biological basis of suture synostosis is currently Morriss-Kay et al. (2001) found that maintenance of pro- poorly understood, but appears to be influenced by a liferating osteogenic stem cells at the margins of mem- combination of vascular, hormonal, genetic, mechanical, brane bones forming the coronal suture requires FGF and local factors (see review in Cohen, 1993). -

Study on Asterion and Presence of Sutural Bones in South Indian Dry Skull
Mohammed Ahad et al /J. Pharm. Sci. & Res. Vol. 7(6), 2015, 390-392 Study on Asterion and Presence of Sutural Bones in South Indian Dry Skull Mohammed Ahad(1),Thenmozhi M.S.(2) 1)BDS 1st year, 2) HOD of Anatomy, Saveetha dental college and hospitals Abstract: Aim: To study morphological features of asterion and presence of sutural bones in posterior side of the 25 human skull. Objective: To know the detailed anatomical knowledge of sutural morphology of asterion and formation of sutural bone. Background: Asterion is the point on Norma lateralis where parietal, temporal and occipital bones meet. It has many neurosurgical importance so any variation during surgery cause damage to dural venous sinuses. Presence of sutural bones will complicate surgical orientation, so it is important to study about the formation of sutural bones and its pattern. Materials and methods: The study will be performed on 25 south Indian dry skull of unknown age and sex taken from the department of anatomy at Saveetha dental college and hospital ,Chennai. Reason: A Research on this topic will lead to the outcome of asterion position from various anatomical landmarks and incidence of sutural bone at posterior side of the skull. Keywords: asterion, sutural bones, surgical importance. INTRODUCTION: The asterion is the junction of the parietal, temporal and occipital bone. It is the surgical landmark to the transverse sinus location, which is of great importance in the surgical approaches to the posterior cranial fossa[1].The sutural morphology was classified into two types: Type 1 where a sutural bone was present and Type 2 where sutural bone was absent.