Rapid Enigmatic Decline Drives the Fire Salamander (Salamandra
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Zoology SYLLABUS 2016
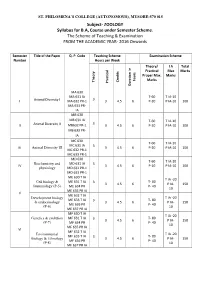
ST. PHILOMENA’S COLLEGE (AUTONOMOUS), MYSORE-570 015 Subject- ZOOLOGY Syllabus for B.A, Course under Semester Scheme. The Scheme of Teaching & Examination FROM THE ACADEMIC YEAR- 2016 Onwards Semester Title of the Paper Q. P. Code Teaching Scheme Examination Scheme Number Hours per Week Theory/ I A Total Practical Max Marks Proper Max. Marks hours Marks Theory Credits Practical Duration in in Duration MA 630 MA 631 IA T-60 T IA-10 3 I Animal Diversity I MA 632 PR-1 3 4.5 6 P-20 P IA-10 100 MA 633 PR- IA MB 630 MB 631 IA T-60 T IA-10 3 II Animal Diversity II MB632 PR-1 3 4.5 6 P-20 P IA-10 100 MB 633 PR- IA MC 630 T-60 T IA-10 MC 631 IA 3 III Animal Diversity III 3 4.5 6 P-20 P IA-10 100 MC 632 PR-1 MC 633 PR-1 MD 630 T-60 T IA-10 Biochemistry and MD 631 IA 3 IV 3 4.5 6 P-20 P IA-10 100 physiology MD 632 PR-1 MD 633 PR-1 ME 630 T IA T IA -20 Cell biology & ME 631 T IA 3 T- 80 3 4.5 6 P IA- 150 Immunology (P-5) ME 634 PR P- 40 10 ME 635 PR IA V ME 632 T IA Development biology T IA -20 ME 633 T IA 3 T- 80 & endocrinology 3 4.5 6 P IA- 150 ME 636 PR P- 40 (P-6) 10 ME 637 PR IA MF 630 T IA T IA -20 Genetics & evolution MF 631 T IA 3 T- 80 3 4.5 6 P IA- 150 (P-7) MF 634 PR P- 40 10 MF 635 PR IA VI MF 632 T IA Environmental T IA -20 MF 633 T IA 3 T- 80 Biology & Ethnology 3 4.5 6 P IA- 150 MF 636 PR P- 40 (P-8) 10 MF 637 PR IA ST. -

Amphibiaweb's Illustrated Amphibians of the Earth
AmphibiaWeb's Illustrated Amphibians of the Earth Created and Illustrated by the 2020-2021 AmphibiaWeb URAP Team: Alice Drozd, Arjun Mehta, Ash Reining, Kira Wiesinger, and Ann T. Chang This introduction to amphibians was written by University of California, Berkeley AmphibiaWeb Undergraduate Research Apprentices for people who love amphibians. Thank you to the many AmphibiaWeb apprentices over the last 21 years for their efforts. Edited by members of the AmphibiaWeb Steering Committee CC BY-NC-SA 2 Dedicated in loving memory of David B. Wake Founding Director of AmphibiaWeb (8 June 1936 - 29 April 2021) Dave Wake was a dedicated amphibian biologist who mentored and educated countless people. With the launch of AmphibiaWeb in 2000, Dave sought to bring the conservation science and basic fact-based biology of all amphibians to a single place where everyone could access the information freely. Until his last day, David remained a tirelessly dedicated scientist and ally of the amphibians of the world. 3 Table of Contents What are Amphibians? Their Characteristics ...................................................................................... 7 Orders of Amphibians.................................................................................... 7 Where are Amphibians? Where are Amphibians? ............................................................................... 9 What are Bioregions? ..................................................................................10 Conservation of Amphibians Why Save Amphibians? ............................................................................. -

About the Book the Format Acknowledgments
About the Book For more than ten years I have been working on a book on bryophyte ecology and was joined by Heinjo During, who has been very helpful in critiquing multiple versions of the chapters. But as the book progressed, the field of bryophyte ecology progressed faster. No chapter ever seemed to stay finished, hence the decision to publish online. Furthermore, rather than being a textbook, it is evolving into an encyclopedia that would be at least three volumes. Having reached the age when I could retire whenever I wanted to, I no longer needed be so concerned with the publish or perish paradigm. In keeping with the sharing nature of bryologists, and the need to educate the non-bryologists about the nature and role of bryophytes in the ecosystem, it seemed my personal goals could best be accomplished by publishing online. This has several advantages for me. I can choose the format I want, I can include lots of color images, and I can post chapters or parts of chapters as I complete them and update later if I find it important. Throughout the book I have posed questions. I have even attempt to offer hypotheses for many of these. It is my hope that these questions and hypotheses will inspire students of all ages to attempt to answer these. Some are simple and could even be done by elementary school children. Others are suitable for undergraduate projects. And some will take lifelong work or a large team of researchers around the world. Have fun with them! The Format The decision to publish Bryophyte Ecology as an ebook occurred after I had a publisher, and I am sure I have not thought of all the complexities of publishing as I complete things, rather than in the order of the planned organization. -

35 Finding of the Alpine Salamander (Salamandra Atra Laurenti, 1768; Salamandridae, Caudata) in the Nature Park Žumberak
HYLA VOL. 2011. No. 1 Str. 35-46 SHORT NOTE Finding of the Alpine salamander (Salamandra atra Laurenti, 1768; Salamandridae, Caudata) in the Nature Park Žumberak - Samoborsko gorje (NW Croatia) 1 2 3 NINA JERAN* , PETRA ĐURIĆ , KREŠIMIR ŽGANEC 1 Požarinje 69, 10000 Zagreb, Croatia, [email protected] 2 State Institute for Nature Protection, Trg Mažuranića 5, 10000 Zagreb, Croatia 3 University of Zagreb, Faculty of Science, Rooseveltov trg 6, 10000 Zagreb, Croatia ABSTRACT This study confirms the presence of Alpine salamander (Salamandra atra) in the Nature Park Žumberak - Samoborsko gorje, where previously only one specimen was recorded in 1989. Species presence and distribution were investigated at ten different localities in stands of montane beech forest, during the vegetation season 2004. In July 2004 five individuals (four males and one female) of Alpine salamander were found in the virgin beech forest at site Kuta (about 900 m a.s.l.), during weather conditions characterized by heavy rain. This is the northernmost finding of the species in Croatia, as well as a confirmed disjunctive part of its areal in the Dinarids. Conservation measures for the species are proposed but for more precise conservation plan further research of species distribution and ecology is needed. Keywords: Alpine salamander, Salamandra atra, Nature Park Žumberak - Samoborsko gorje, distribution, amphibians 35 Alpine salamander (Salamandra atra) is a montane species occurring between 400 and 2,800 m a.s.l., but is usually found from 800 to 2,000 m a.s.l. (ARNOLD & BURTON, 1978). It inhabits mainly forests (beech, mixed deciduous and mixed deciduous-coniferous), but may also be found above the tree-line, in cool, damp alpine meadows, pastureland and other, slightly modified habitats. -

MAHS Care Sheet Master List *By Eric Roscoe Care Sheets Are Often An
MAHS Care Sheet Master List *By Eric Roscoe Care sheets are often an excellent starting point for learning more about the biology and husbandry of a given species, including their housing/enclosure requirements, temperament and handling, diet , and other aspects of care. MAHS itself has created many such care sheets for a wide range of reptiles, amphibians, and invertebrates we believe to have straightforward care requirements, and thus make suitable family and beginner’s to intermediate level pets. Some species with much more complex, difficult to meet, or impracticable care requirements than what can be adequately explained in a one page care sheet may be multiple pages. We can also provide additional links, resources, and information on these species we feel are reliable and trustworthy if requested. If you would like to request a copy of a care sheet for any of the species listed below, or have a suggestion for an animal you don’t see on our list, contact us to let us know! Unfortunately, for liability reasons, MAHS is unable to create or publish care sheets for medically significant venomous species. This includes species in the families Crotilidae, Viperidae, and Elapidae, as well as the Helodermatidae (the Gila Monsters and Mexican Beaded Lizards) and some medically significant rear fanged Colubridae. Those that are serious about wishing to learn more about venomous reptile husbandry that cannot be adequately covered in one to three page care sheets should take the time to utilize all available resources by reading books and literature, consulting with, and working with an experienced and knowledgeable mentor in order to learn the ropes hands on. -

The Salamanders of Tennessee
Salamanders of Tennessee: modified from Lisa Powers tnwildlife.org Follow links to Nongame The Salamanders of Tennessee Photo by John White Salamanders are the group of tailed, vertebrate animals that along with frogs and caecilians make up the class Amphibia. Salamanders are ectothermic (cold-blooded), have smooth glandular skin, lack claws and must have a moist environment in which to live. 1 Amphibian Declines Worldwide, over 200 amphibian species have experienced recent population declines. Scientists have reports of 32 species First discovered in 1967, the golden extinctions, toad, Bufo periglenes, was last seen mainly species of in 1987. frogs. Much attention has been given to the Anurans (frogs) in recent years, however salamander populations have been poorly monitored. Photo by Henk Wallays Fire Salamander - Salamandra salamandra terrestris 2 Why The Concern For Salamanders in Tennessee? Their key role and high densities in many forests The stability in their counts and populations Their vulnerability to air and water pollution Their sensitivity as a measure of change The threatened and endangered status of several species Their inherent beauty and appeal as a creature to study and conserve. *Possible Factors Influencing Declines Around the World Climate Change Habitat Modification Habitat Fragmentation Introduced Species UV-B Radiation Chemical Contaminants Disease Trade in Amphibians as Pets *Often declines are caused by a combination of factors and do not have a single cause. Major Causes for Declines in Tennessee Habitat Modification -The destruction of natural habitats is undoubtedly the biggest threat facing amphibians in Tennessee. Housing, shopping center, industrial and highway construction are all increasing throughout the state and consequently decreasing the amount of available habitat for amphibians. -

Fire Salamander Care Sheet
Fire salamander Care Sheet Common names: Fire Salamander It is believed that Fire Salamanders received their name by hiding in logs chopped for burning and then running out once the logs were placed on the fire. A myth grew up over this habit and our ancestors believed that they could not only withstand fire, but also that they lived in it. Scientific Name : Salamandra salamandra There are two main sub-species that are commonly found in the pet trade. These are Salamandra salamandra salamandra and Salamandra salamandra terrestis . S.s terrestis is sometimes called the Barred Fire Salamander. This care sheet will focus on these two species, but here is a list of some other Salamanders in the Salamandra salamandra family: Spotted Fire Salamander - Salamandra salamandra almanzoris Yellow Striped Fire Salamander - Salamandra salamandra fastuosa Bernadezi Fire Salamander - Salamandra salamandra bernadezi Near Eastern Fire Salamander - Samandra salamandra inframmaculata Portuguese Fire Salamander - Salamndra salamandra gallaica Corsican Fire Salamander - Salamandra salamandra corsica Los Barrios Fire Salamander - Salamandra salamandra longirostris Description Description: Depending on the type of Fire Salamander you have, their dorsum colours tend to be a glossy black with bright yellow blotches on their bodies. This yellow colouration varies between the species and is usually the way that the sub-species are differentiated from each other. They are quite stocky salamanders with long tails and visible parotoid glands in the area behind their protruding eyes. Size: Fire Salamanders generally grow to between 18 to 25cms (7-10inches) in length, but it is not unknown for them to reach 30cms (12inch) in some cases. -

The Central Texas Master Naturalist Newsletter December 2017
The Tracker The Central Texas Master Naturalist Newsletter December 2017 Mother Neff State Park Association Inside This Issue Chautauqua Fundraiser President’s Pen 3 -Rene Berkhoudt Mother Neff Chautauqua 1 The Mother Neff State Park Salado Sirena Fest 5 Association held its annual Chautauqua fundraiser this year Macroinvertebrates 7 on November 4th. You may well Autumn Youth Hikes 8 ask what the word ‘Chautauqua’ means. October Awards 9 The first Chautauqua, the New Holiday Cheer 11 York Chautauqua Assembly, was organized in 1874 at a campsite Scaling Down Nature 12 on the shores of Chautauqua Interesting Tidbits 13 Lake in New York State. Chautauqua lent its name to an adult education movement in the Calendar at a Glance United States that was highly popular in the late 19th and early December 5, hours due. 20th centuries. The Chautauqua was culture and entertainment for December 9, Mammoths in the the whole community, with speakers, teachers, musicians, entertainers, Moonlight— Mammoth National preachers, and specialists of the day. Former U.S. President Theodore Monument — Waco Roosevelt was quoted as saying that Chautauqua is "the most American December 12, 6:30 pm, 8th thing in America". The Chautauqua began to decline as a public forum Annual CTMN Holiday Party— in the 1920s in part due to the introduction of radio broadcasting. Zoe Rascoe’s House December 16, Winter Solstice — Chapter Motto Cave Without a Name—Boerne Earth Day, Continued on page 14... Every Day The Mother Neff State Park Association Chautauqua was a celebration of autumn and of all the wonderful things this beautiful park has to offer and featured a variety of activities, demonstrations and displays from diverse groups; such as the Buffalo Soldiers of the 9th and 10th U.S. -

Conservation and Ecology of the Endangered Fire Salamander (Salamandra Infraimmaculata) Ori Segev a THESIS SUBMITTED for THE
Conservation and ecology of the endangered fire salamander ( Salamandra infraimmaculata ) Ori Segev A THESIS SUBMITTED FOR THE DEGREE "DOCTOR OF PHILOSOPHY" University of Haifa Faculty of Science and Science Education Department of Evolution and Environmental Biology NOVEMBER, 2009 Conservation and ecology of the endangered fire salamander ( Salamandra infraimmaculata ) By: Ori Segev Supervised by: Leon Blaustein A THESIS SUBMITTED FOR THE DEGREE "DOCTOR OF PHILOSOPHY" University of Haifa Faculty of Science and Science Education Department of Evolution and Environmental Biology NOVEMBER, 2009 Recommended by: ________________ Date: _____________ (Advisor) Approved by: ____________________ Date: _____________ (Chairman of Ph.D. Committee) i Acknowledgments ii Table of contents Abstract ………………………………………………………………….…..................…vii List of Tables ………...………………..……………………………………………........…x List of Figures ………………………………...…….……………………..….……….….xii General Introduction ……………..…………………………….……...............................1 Amphibian decline …………………………………………………………………..1 Salamandra distribution and conservation status ………………………………......2 Population size and breeding phenology ………………...……………...........…….3 Invasive species ……………………..…………………………………...………….4 Breeding strategies and habitat selection ………………………………...........…...5 Priority effects ………………………………………………………...……….……6 Chapter 1. Population size, structure and phenology of an endangered salamander at temporary and permanent breeding sites .........................8-29 Abstract...............................................................................................................9 -

SALAMANDER CHYTRIDIOMYCOSIS Other Names: Salamander Chytrid Disease, B Sal
SALAMANDER CHYTRIDIOMYCOSIS Other names: salamander chytrid disease, B sal CAUSE Salamander chytridiomycosis is an infectious disease caused by the fungus Batrachochytrium salamandrivorans. The fungus is a close relative of B. dendrobatidis, which was described more than two decades ago and is responsible for the decline or extinction of over 200 species of frogs and toads. Salamander chytridiomycosis, and the fungus that causes it, were only recently discovered. The first cases occurred in The Netherlands, as outbreaks in native fire salamanders, Salamandra salamandra. Further work discovered that the fungus is present in Thailand, Vietnam and Japan, and can infect native Eastern Asian salamanders without causing significant disease. Evidence suggests that the fungus was introduced to Europe in the last decade or so, probably through imported exotic salamanders that can act as carriers. Once introduced the fungus is capable of surviving in the environment, amongst the leaf litter and in small water bodies, even in the absence of salamanders. It thrives at temperatures between 10-15°C, with some growth in temperatures as low as 5°C and death at 25°C. B. salamandrivorans has not, so far, been reported in North America. SIGNIFICANCE The disease is not present in North America, but an introduction of the fungus into native salamander populations could have devastating effects. In Europe, the fire salamander population where the disease was first discovered is at the brink of extirpation, with over 96% mortality recorded during outbreaks. Little is known about the susceptibility of most North American salamanders but, based on experimental trials, at least two species, the Eastern newt (Notophthalmus viridescens) and the rough-skinned newt (Taricha granulosa), are highly susceptible to the fungus and could experience similar high mortalities. -

Salamander Species Listed As Injurious Wildlife Under 50 CFR 16.14 Due to Risk of Salamander Chytrid Fungus Effective January 28, 2016
Salamander Species Listed as Injurious Wildlife Under 50 CFR 16.14 Due to Risk of Salamander Chytrid Fungus Effective January 28, 2016 Effective January 28, 2016, both importation into the United States and interstate transportation between States, the District of Columbia, the Commonwealth of Puerto Rico, or any territory or possession of the United States of any live or dead specimen, including parts, of these 20 genera of salamanders are prohibited, except by permit for zoological, educational, medical, or scientific purposes (in accordance with permit conditions) or by Federal agencies without a permit solely for their own use. This action is necessary to protect the interests of wildlife and wildlife resources from the introduction, establishment, and spread of the chytrid fungus Batrachochytrium salamandrivorans into ecosystems of the United States. The listing includes all species in these 20 genera: Chioglossa, Cynops, Euproctus, Hydromantes, Hynobius, Ichthyosaura, Lissotriton, Neurergus, Notophthalmus, Onychodactylus, Paramesotriton, Plethodon, Pleurodeles, Salamandra, Salamandrella, Salamandrina, Siren, Taricha, Triturus, and Tylototriton The species are: (1) Chioglossa lusitanica (golden striped salamander). (2) Cynops chenggongensis (Chenggong fire-bellied newt). (3) Cynops cyanurus (blue-tailed fire-bellied newt). (4) Cynops ensicauda (sword-tailed newt). (5) Cynops fudingensis (Fuding fire-bellied newt). (6) Cynops glaucus (bluish grey newt, Huilan Rongyuan). (7) Cynops orientalis (Oriental fire belly newt, Oriental fire-bellied newt). (8) Cynops orphicus (no common name). (9) Cynops pyrrhogaster (Japanese newt, Japanese fire-bellied newt). (10) Cynops wolterstorffi (Kunming Lake newt). (11) Euproctus montanus (Corsican brook salamander). (12) Euproctus platycephalus (Sardinian brook salamander). (13) Hydromantes ambrosii (Ambrosi salamander). (14) Hydromantes brunus (limestone salamander). (15) Hydromantes flavus (Mount Albo cave salamander). -

Invasive Species Alert! Salamander Chytrid Disease
INVASIVE SPECIES ALERT! SALAMANDER CHYTRID DISEASE REPORT INVASIVE SPECIES (Batrachochytrium salamandrivorans) www.reportinvasives.ca NATIVE RANGE Bsal is believed to be endemic to Asia. DESCRIPTION Bsal... Affects salamanders and newts Causes reddening, ulcerations, and excessive shedding on salamander and newt skin Can lead to skin lesions, anorexia, lethargic, ataxia, and eventual mortality WHY SHOULD WE CARE? PRIMARY IMPACT: Bsal There are nine species of salamanders and newts that live in BC, four of which are listed under the Species at Risk Act as is a fungus that causes endangered, threatened or special concern Salamander Research has shown that species of pacific North American salamanders and newts are at high risk of contracting Bsal, as it Chytridiomycocis, a could establish in the province disease that has been A decline in salamanders and newts could greatly alter ecosystem dynamics because: causing mass declines in o Salamanders and newts are predators of insects and Fire Salamander other arthropods and may contribute to regulating population numbers of these groups (Salamandra salamandra) o Salamanders and newts are prey for other species, thus populations in Europe. playing an important role within the food chain Salamander skin contains complex antibacterial and antifungal compounds and toxins that could one day provide benefits to humans that scientists are simply unaware of at this time DID YOU KNOW? Salamanders are believed to be an excellent indicator species for Members of the public (including scientists to monitor ecosystem health boaters, fishers, and other recreational users) and researchers BIOLOGY & SPREAD that enter into Bsal infected or The spread of Bsal to salamanders and newts occurs through direct potentially infected sites are urged to contact with an infected individual or with water or organic material that follow proper hygiene protocols; an infected individual previously contacted.