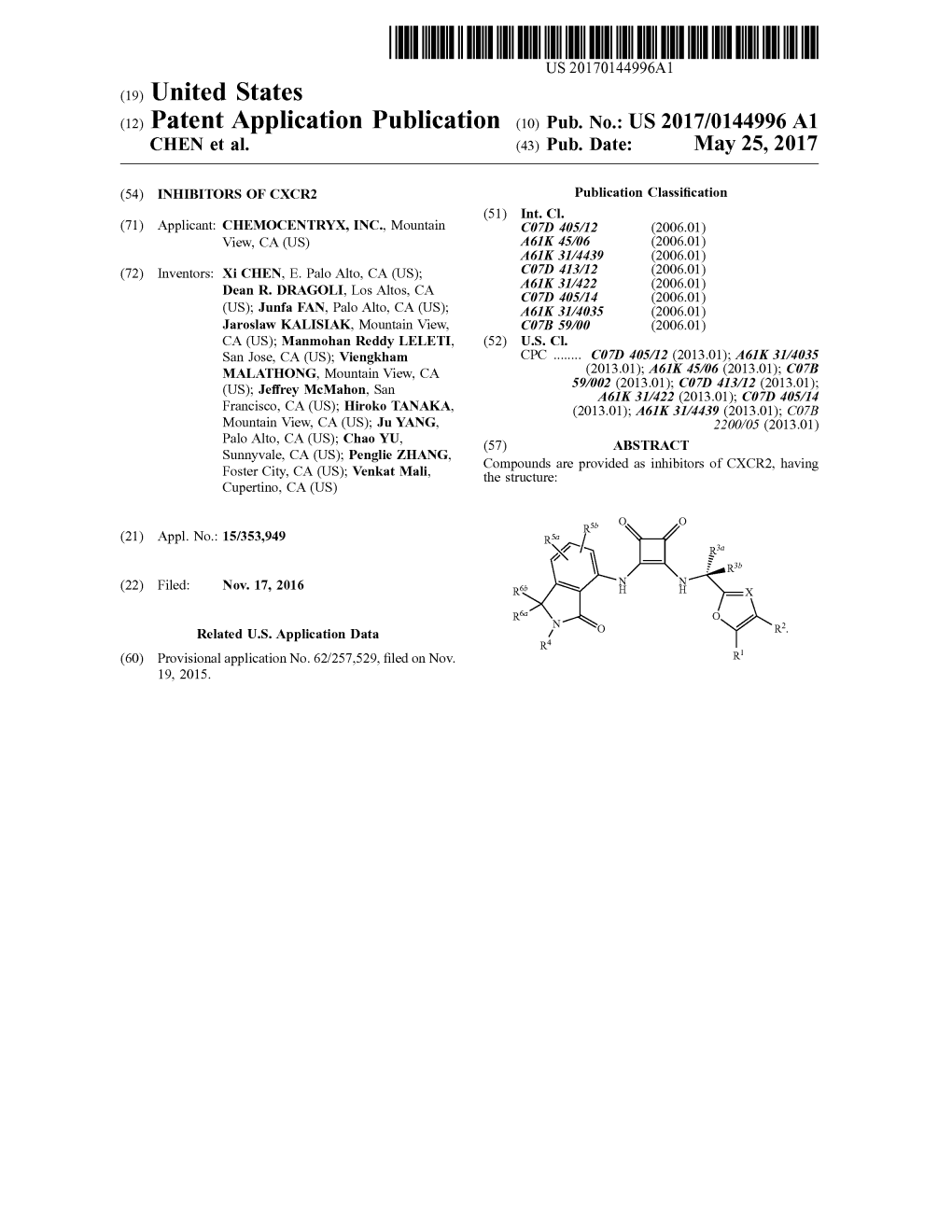
(12) Patent Application Publication (10) Pub. No.: US 2017/0144996 A1 CHEN Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

S1 Table. List of Medications Analyzed in Present Study Drug
S1 Table. List of medications analyzed in present study Drug class Drugs Propofol, ketamine, etomidate, Barbiturate (1) (thiopental) Benzodiazepines (28) (midazolam, lorazepam, clonazepam, diazepam, chlordiazepoxide, oxazepam, potassium Sedatives clorazepate, bromazepam, clobazam, alprazolam, pinazepam, (32 drugs) nordazepam, fludiazepam, ethyl loflazepate, etizolam, clotiazepam, tofisopam, flurazepam, flunitrazepam, estazolam, triazolam, lormetazepam, temazepam, brotizolam, quazepam, loprazolam, zopiclone, zolpidem) Fentanyl, alfentanil, sufentanil, remifentanil, morphine, Opioid analgesics hydromorphone, nicomorphine, oxycodone, tramadol, (10 drugs) pethidine Acetaminophen, Non-steroidal anti-inflammatory drugs (36) (celecoxib, polmacoxib, etoricoxib, nimesulide, aceclofenac, acemetacin, amfenac, cinnoxicam, dexibuprofen, diclofenac, emorfazone, Non-opioid analgesics etodolac, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, (44 drugs) ketoprofen, ketorolac, lornoxicam, loxoprofen, mefenamiate, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, pranoprofen, proglumetacin, sulindac, talniflumate, tenoxicam, tiaprofenic acid, zaltoprofen, morniflumate, pelubiprofen, indomethacin), Anticonvulsants (7) (gabapentin, pregabalin, lamotrigine, levetiracetam, carbamazepine, valproic acid, lacosamide) Vecuronium, rocuronium bromide, cisatracurium, atracurium, Neuromuscular hexafluronium, pipecuronium bromide, doxacurium chloride, blocking agents fazadinium bromide, mivacurium chloride, (12 drugs) pancuronium, gallamine, succinylcholine -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

Download Product Insert (PDF)
PRODUCT INFORMATION Polmacoxib Item No. 17509 OO CAS Registry No.: 301692-76-2 Formal Name: 4-[3-(3-fluorophenyl)-4,5-dihydro-5,5-dimethyl- S NH 4-oxo-2-furanyl]-benzenesulfonamide 2 MF: C H FNO S 18 16 4 O FW: 361.4 Purity: ≥98% F UV/Vis.: λmax: 238, 320 nm Supplied as: A crystalline solid O Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Polmacoxib is supplied as a crystalline solid. A stock solution may be made by dissolving the polmacoxib in the solvent of choice. Polmacoxib is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF), which should be purged with an inert gas. The solubility of polmacoxib in ethanol is approximately 5 mg/ml and approximately 20 mg/ml in DMSO and DMF. Polmacoxib is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, polmacoxib should first be dissolved in DMSO and then diluted with the aqueous buffer of choice. Polmacoxib has a solubility of approximately 0.5 mg/ml in a 1:8 solution of DMSO:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day. Description Polmacoxib is an inhibitor of cyclooxygenase 2 (COX-2) and the carbonic anhydrase subtypes I (CAI) and 1 CAII. It inhibits COX-2 in the absence of carbonic anhydrase II with an IC50 value of 40 nM, which increases by approximately 4- and 17-fold in the presence of a CAII at a molar ratio of 1:1 and 1:5, respectively.2 It also inhibits CAI and CAII (IC50s = 210 and 95 nM, respectively). -

Analgesic & Rheumatology
Recommendations of the SEC (Analgesic & Rheumatology) made in its 72nd meeting held on 24.06.2021 & 25.06.2021 at CDSCO HQ New Delhi. File Name & Recommendations Agenda Drug Name, Firm Name No Strength New Drugs Division ND/MA/21/000076 M/s Hetero Labs Ltd. The firm presented their proposal of Polmacoxib 2 mg Fresh Application Phase III clinical trial protocol capsules along with BE study protocol before committee. After detailed deliberation, the 1. committee recommended for grant of permission to conduct the BE study. The firm should present the results of BE study before committee for further consideration of phase III clinical trial protocol. Biological Division BIO/CT- The firm presented their proposal 18/FF/2021/24609- for marketing authorization and Belimumab (Marketing Authorization)- local clinical trial waiver based on Belimumab the results of GCT including sub set analysis on 66 Indian patients. The committee noted that the drug is not an unmet need in the country. M/s. GSK Further, the data presented by the 2. Pharmaceuticals firm in Indian sub-set is limited and Ltd. in different route of administration (intravenous) while the firm is seeking MA for subcutaneous route. After detailed deliberation, the committee did not recommend for grant of approval for marketing authorization. BIO/CT21/FF/2021/246 M/s Reliance Life The firm presented their proposal 79- Denosumab Sciences Pvt ltd for marketing authorization along 60mg/ml with the results of Phase-III clinical trial. 3. The committee noted that the Phase III trial was approved -

Concomitant Use of Nsaids Or Ssris with Noacs Requires Monitoring for Bleeding
Original Article Yonsei Med J 2020 Sep;61(9):741-749 https://doi.org/10.3349/ymj.2020.61.9.741 pISSN: 0513-5796 · eISSN: 1976-2437 Concomitant Use of NSAIDs or SSRIs with NOACs Requires Monitoring for Bleeding Min-Taek Lee1, Kwang-Yeol Park2, Myo-Song Kim1, Seung-Hun You1, Ye-Jin Kang1, and Sun-Young Jung1 1College of Pharmacy, Chung-Ang University, Seoul; 2Department of Neurology, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Korea. Purpose: Non-vitamin K antagonist oral anticoagulants (NOACs) are widely used in patients with atrial fibrillation (AF) because of their effectiveness in preventing stroke and their better safety, compared with warfarin. However, there are concerns for an in- creased risk of bleeding associated with concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs) or selective sero- tonin reuptake inhibitors (SSRIs) with NOACs. In this study, we aimed to evaluate the risk of bleeding events in individuals taking concomitant NSAIDs or SSRIs with NOACs after being diagnosed with AF. Materials and Methods: A nested case-control analysis to assess the safety of NSAIDs and SSRIs among NOAC users with AF was performed using data from Korean National Health Insurance Service from January 2012 to December 2017. Among patients who were newly prescribed NOACs, 1233 cases hospitalized for bleeding events were selected, and 24660 controls were determined. Results: The risk of bleeding events was higher in patients receiving concomitant NSAIDs [adjusted odds ratio (aOR) 1.41; 95% confidence interval (CI) 1.24–1.61] or SSRIs (aOR 1.92; 95% CI 1.52–2.42) with NOACs, compared to no use of either drug, respec- tively. -

Directory of Korean Pharmaceutical Industry 2016
2016 Directory of Korean Pharmaceutical Industry Contents 1. Greeting ������������������������������������������������������������������������������������������������������������������������������������������� 4 2. Overview of the Korean Pharmaceutical Industry ������������������������������������������������������������ 6 1) Current Status 2) Production Management System 3) Development of New Drugs 4) R&D Reinforcement 5) Biopharmaceuticals 6) Globalization 7) Korean Pharmaceutical Industry Vision - PHARMA 2020 3. Directory of Pharmaceutical Companies �������������������������������������������������������������������������� 13 · Regular Members(2015. 4) A~D ���������������������������������������������������������������������������������������������������������������������������������������������������������������������������� 14 E~J ��������������������������������������������������������������������������������������������������������������������������������������������������������������������������� 66 K~P �������������������������������������������������������������������������������������������������������������������������������������������������������������������������� 110 R~Y �������������������������������������������������������������������������������������������������������������������������������������������������������������������������� 158 · Associated Members(2015. 4) C~G ��������������������������������������������������������������������������������������������������������������������������������������������������������������������������� -

“M/S. AJANTA PHARMA LTD.”
APPLICATION FOR ENVIRONMENTAL CLEARANCE OF PROPOSED EXPANSION OF ACTIVE PHARMACEUTICAL INGREDIENTS (API), MANUFACTURING UNIT “M/s. AJANTA PHARMA LTD.” 11KM Stone, Gut No. 378, Plot No 8, Aurangabad –Pune Highway, Village-Waluj, Taluka. Gangapur, District. Aurangabad- 431133. FORM 1 Submitted to Expert Appraisal Committee (Industry-2), MoEFCC, New Delhi Submitted by M/s. AJANTA PHARMA LTD. Environmental Consultant: Building Environment (India) Pvt. Ltd Dakshina Building, Office No. 401, Plot No. 2, Sector-11, CBD Belapur, Navi Mumbai- 400 614 January, 2018. Form 1 for proposed Expansion of API Manufacturing Industry “M/s. Ajanta Pharma Ltd.” at Waluj Village, Taluka-Gangapur, District- Aurangabad, Maharashtra. Form – 1 (I) Basic Information:- Sr. Items Details No. 1. Name of the project Proposed expansion of Active Pharmaceutical Ingredients (API) Manufacturing Industry “M/s. Ajanta Pharma Ltd.” 2. S. No. in the schedule Category 5f as per EIA Notification 2006 & amendments 3. Proposed capacity/ area/ length/ Industry is already engaged in manufacturing 85 nos. of tonnage to be handled/ command API and having production capacity 21.042 MT/Month. area/lease area/ number of wells to In the proposed expansion industry will manufacture 256 be drilled nos. API including existing API. The total production capacity after expansion would remain same as existing i.e. 21.042 MT/Month. Annexure-1 : Details of existing & proposed API 4. New / Expansion / Modernization Expansion. Annexure -2: EC letter of existing unit 5. Existing Capacity/ Area etc. Industry is already engaged in manufacturing 85 nos. of API and having production 21.042 MT/Month. Annexure-3 : Details of existing API 6. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

Selection of Analgesics for the Management of Acute And
J Periodontal Implant Sci. 2020 Apr;50(2):68-73 https://doi.org/10.5051/jpis.2020.50.2.68 pISSN 2093-2278·eISSN 2093-2286 Review Selection of analgesics for Periodontal Science the management of acute and postoperative dental pain: a mini-review Sung-Jin Kim 1,2, Jeong Taeg Seo 1,* 1Department of Oral Biology, Yonsei University College of Dentistry, Seoul, Korea 2Department of Oral Biology, BK21 PLUS Project, Yonsei University College of Dentistry, Seoul, Korea Received: Oct 14, 2019 ABSTRACT Revised: Jan 5, 2020 Accepted: Feb 12, 2020 Pain management is an important part of dental practice, and dentists frequently prescribe *Correspondence: analgesics to improve clinical outcomes. Dentists should be aware of the pharmacological Jeong Taeg Seo characteristics of the analgesics commonly used in dentistry and should choose appropriate Department of Oral Biology, Yonsei University analgesics to treat and prevent pain associated with inflammation or surgery. In this article, College of Dentistry, Yonsei-ro 50-1, Seodaemun-gu, Seoul 03722, Korea. we review the potential benefits and risks of the analgesics frequently used in dental practice E-mail: [email protected] and provide a stepwise approach for pain management. Tel: +82-2-2228-3054 Keywords: Acetaminophen; Analgesics; Ibuprofen; Naproxen; Copyright © 2020. Korean Academy of Non-steroidal anti-inflammatory drugs Periodontology This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https:// INTRODUCTION creativecommons.org/licenses/by-nc/4.0/). ORCID iDs Effective and safe pain management is a primary goal in dental practice. Control of pain Sung-Jin Kim associated with dental or periodontal disease is a main reason that patients seek care from https://orcid.org/0000-0003-4115-0403 dentists. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of - Medicines belonging to the ATC group M01 (Antiinflammatory and antirheumatic products) Table of Contents Page INTRODUCTION 6 DISCLAIMER 8 GLOSSARY OF TERMS USED IN THIS DOCUMENT 9 ACTIVE SUBSTANCES Phenylbutazone (ATC: M01AA01) 11 Mofebutazone (ATC: M01AA02) 17 Oxyphenbutazone (ATC: M01AA03) 18 Clofezone (ATC: M01AA05) 19 Kebuzone (ATC: M01AA06) 20 Indometacin (ATC: M01AB01) 21 Sulindac (ATC: M01AB02) 25 Tolmetin (ATC: M01AB03) 30 Zomepirac (ATC: M01AB04) 33 Diclofenac (ATC: M01AB05) 34 Alclofenac (ATC: M01AB06) 39 Bumadizone (ATC: M01AB07) 40 Etodolac (ATC: M01AB08) 41 Lonazolac (ATC: M01AB09) 45 Fentiazac (ATC: M01AB10) 46 Acemetacin (ATC: M01AB11) 48 Difenpiramide (ATC: M01AB12) 53 Oxametacin (ATC: M01AB13) 54 Proglumetacin (ATC: M01AB14) 55 Ketorolac (ATC: M01AB15) 57 Aceclofenac (ATC: M01AB16) 63 Bufexamac (ATC: M01AB17) 67 2 Indometacin, Combinations (ATC: M01AB51) 68 Diclofenac, Combinations (ATC: M01AB55) 69 Piroxicam (ATC: M01AC01) 73 Tenoxicam (ATC: M01AC02) 77 Droxicam (ATC: M01AC04) 82 Lornoxicam (ATC: M01AC05) 83 Meloxicam (ATC: M01AC06) 87 Meloxicam, Combinations (ATC: M01AC56) 91 Ibuprofen (ATC: M01AE01) 92 Naproxen (ATC: M01AE02) 98 Ketoprofen (ATC: M01AE03) 104 Fenoprofen (ATC: M01AE04) 109 Fenbufen (ATC: M01AE05) 112 Benoxaprofen (ATC: M01AE06) 113 Suprofen (ATC: M01AE07) 114 Pirprofen (ATC: M01AE08) 115 Flurbiprofen (ATC: M01AE09) 116 Indoprofen (ATC: M01AE10) 120 Tiaprofenic Acid (ATC: -

Association Between Nsaids Use and Adverse Clinical Outcomes Among Adults Hospitalised with COVID-19 in South Korea: a Nationwide Study
medRxiv preprint doi: https://doi.org/10.1101/2020.06.01.20119768; this version posted June 16, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Association between NSAIDs use and adverse clinical outcomes among adults hospitalised with COVID-19 in South Korea: A nationwide study Han Eol Jeong MPH,1, † Hyesung Lee MS,1, † Hyun Joon Shin MD,2 Young June Choe MD,3 Kristian B. Filion PhD,4,5 Ju-Young Shin PhD1,6 †These authors contributed equally to this work as co-first authors 1 School of Pharmacy, Sungkyunkwan University, Suwon, Gyeonggi-do, South Korea 2 Division of General Internal Medicine, Department of Medicine, Brigham and Women's Hospital, Department of Global Health and Social Medicine, Harvard Medical School, Boston, MA, USA. 3 Division of Infectious Diseases, Department of Social and Preventive Medicine, Hallym University College of Medicine, Chuncheon, Gangwon-do, South Korea 4 Departments of Medicine and Epidemiology, Biostatistics and Occupational Health, McGill University, Montreal, Quebec, Canada. 5 Centre for Clinical Epidemiology, Lady Davis Institute, Montreal, Quebec, Canada. 6 Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul, South Korea Word count (summary): 308 Word count (main text): 3,061 Corresponding author: Dr Ju-Young Shin School of Pharmacy, Sungkyunkwan University, 2066, Seobu-ro, Jangan-gu, Suwon, Gyeonggi-do 16419, South Korea Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, 81 Irwon-ro, Gangnam-gu, Seoul 06351, South Korea Tel: +82-31-290-7702; E-mail: [email protected] 1 NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. -

(12) Patent Application Publication (10) Pub. No.: US 2017/0144997 A1 CHEN Et Al
US 201701.44997 A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0144997 A1 CHEN et al. (43) Pub. Date: May 25, 2017 (54) MODULATORS OF CHEMOKINE A6II 45/06 (2006.01) RECEPTORS C07D 405/2 (2006.01) A6II 3/4725 (2006.01) (71) Applicant: CHEMOCENTRYX, INC., Mountain A63L/455 (2006.01) View, CA (US) A6II 3/4439 (2006.01) C07D 209/246 (2006.01) (72) Inventors: Xi CHEN, E. Palo Alto, CA (US); A63L/506 (2006.01) Dean R. DRAGOLI, Los Altos, CA A63L/454 (2006.01) (US); Junfa FAN, Palo Alto, CA (US); C07D 307/52 (2006.01) Jaroslaw KALISAK, Mountain View, A63L/4035 (2006.01) CA (US); Antoni KRASINSKI, (52) U.S. C. Sunnyvale, CA (US); Manmohan CPC ......... C07D405/14 (2013.01); C07D 307/52 Reddy Leleti, San Jose, CA (US); (2013.01); A61 K3I/341 (2013.01); A61 K Venkat MALI, Cupertino, CA (US); 45/06 (2013.01); C07D405/12 (2013.01); Jeffrey McMAHON, San Francisco, A61K 31/4035 (2013.01); A61K 31/4155 CA (US); Rajinder SINGH, Belmont, (2013.01); A61 K3I/4439 (2013.01); C07D CA (US); Hiroko TANAKA, Mountain 209/46 (2013.01); A61 K3I/506 (2013.01); View, CA (US); Ju YANG, Palo Alto, A6 IK3I/454 (2013.01); A61K 31/4725 CA (US); Chao YU, Sunnyvale, CA (2013.01) (US); Penglie ZHANG, Foster City, CA (US) (57) ABSTRACT Compounds are provided as chemokine inhibitors having (21) Appl. No.: 15/353,889 the structure: (22) Filed: Nov. 17, 2016 Related U.S. Application Data R5a R55 O O R7 (60) Provisional application No.