Polygonum Aviculare
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stace Edition 4: Changes
STACE EDITION 4: CHANGES NOTES Changes to the textual content of keys and species accounts are not covered. "Mention" implies that the taxon is or was given summary treatment at the head of a family, family division or genus (just after the key if there is one). "Reference" implies that the taxon is or was given summary treatment inline in the accounts for a genus. "Account" implies that the taxon is or was given a numbered account inline in the numbered treatments within a genus. "Key" means key at species / infraspecific level unless otherwise qualified. "Added" against an account, mention or reference implies that no treatment was given in Edition 3. "Given" against an account, mention or reference implies that this replaces a less full or prominent treatment in Stace 3. “Reduced to” against an account or reference implies that this replaces a fuller or more prominent treatment in Stace 3. GENERAL Family order changed in the Malpighiales Family order changed in the Cornales Order Boraginales introduced, with families Hydrophyllaceae and Boraginaceae Family order changed in the Lamiales BY FAMILY 1 LYCOPODIACEAE 4 DIPHASIASTRUM Key added. D. complanatum => D. x issleri D. tristachyum keyed and account added. 5 EQUISETACEAE 1 EQUISETUM Key expanded. E. x meridionale added to key and given account. 7 HYMENOPHYLLACEAE 1 HYMENOPHYLLUM H. x scopulorum given reference. 11 DENNSTAEDTIACEAE 2 HYPOLEPIS added. Genus account added. Issue 7: 26 December 2019 Page 1 of 35 Stace edition 4 changes H. ambigua: account added. 13 CYSTOPTERIDACEAE Takes on Gymnocarpium, Cystopteris from Woodsiaceae. 2 CYSTOPTERIS C. fragilis ssp. fragilis: account added. -

Environmental Weeds of Coastal Plains and Heathy Forests Bioregions of Victoria Heading in Band
Advisory list of environmental weeds of coastal plains and heathy forests bioregions of Victoria Heading in band b Advisory list of environmental weeds of coastal plains and heathy forests bioregions of Victoria Heading in band Advisory list of environmental weeds of coastal plains and heathy forests bioregions of Victoria Contents Introduction 1 Purpose of the list 1 Limitations 1 Relationship to statutory lists 1 Composition of the list and assessment of taxa 2 Categories of environmental weeds 5 Arrangement of the list 5 Column 1: Botanical Name 5 Column 2: Common Name 5 Column 3: Ranking Score 5 Column 4: Listed in the CALP Act 1994 5 Column 5: Victorian Alert Weed 5 Column 6: National Alert Weed 5 Column 7: Weed of National Significance 5 Statistics 5 Further information & feedback 6 Your involvement 6 Links 6 Weed identification texts 6 Citation 6 Acknowledgments 6 Bibliography 6 Census reference 6 Appendix 1 Environmental weeds of coastal plains and heathy forests bioregions of Victoria listed alphabetically within risk categories. 7 Appendix 2 Environmental weeds of coastal plains and heathy forests bioregions of Victoria listed by botanical name. 19 Appendix 3 Environmental weeds of coastal plains and heathy forests bioregions of Victoria listed by common name. 31 Advisory list of environmental weeds of coastal plains and heathy forests bioregions of Victoria i Published by the Victorian Government Department of Sustainability and Environment Melbourne, March2008 © The State of Victoria Department of Sustainability and Environment 2009 This publication is copyright. No part may be reproduced by any process except in accordance with the provisions of the Copyright Act 1968. -

CURLY DOCK SHEEP SORREL Rumex Crisp Us Rumex Acetosella
CURLY DOCK SHEEP SORREL Rumex crisp us Rumex acetosella FAMILY: Polygonaceae GENUS: Rumex SPECIES: crispus acetosella COMMON NAMES: Curly Dock Sheep Sorrel Yellow Dock Sorrell Dock Sour Dock Field Sorrel Narrow-leaved Dock Red Sorrel Wood Sorrel Sour-weed POLLEN GRAINS: Spheroidal, 23 to 27 microns in diameter. A thin exine is finely pitted. Three or four (sometimes six) linear furrows are evenly arranged, each having a small elliptical germ pore. POLLINATING PERIOD: June to August. Earlier in warmer Throughout the summer but areas. heaviest in May and June. DISTRIBUTION: Throughout the United States. ThroughoutmostoftheUnited States. Rarely found in Southern California or desert areas of the Southwest. ALLERGIC IMPORTANCE: Of only moderate Importance In areas of abundance. Curly Dock is a stout perennial 1 ~ to 5 feet Sheep Sorrel is a perennial ~ to 2 feet tall tall. During the first year the plant forms a arising from a slender running rootstock. The dense roseaffe of leaves. Leaves are elon leaves are rather fleshy and vary in size and gated with wavy margins. The upright stems shape from lower to upper. The lower leaves are leafy. The terminal inflorescence is dense are halbard-shaped with two basal lobes. with few or no leaves and is 1 to 2 feet long. In The inflorescence is terminal and mostly leaf fruit in the late summer and fall the tops turn a less. Individual flowers are of separate sex characteristic reddish brown. with the female being red and the male a yellowish green color. The foliage of the docks is acid but especially so in this species. -

Somerset's Ecological Network
Somerset’s Ecological Network Mapping the components of the ecological network in Somerset 2015 Report This report was produced by Michele Bowe, Eleanor Higginson, Jake Chant and Michelle Osbourn of Somerset Wildlife Trust, and Larry Burrows of Somerset County Council, with the support of Dr Kevin Watts of Forest Research. The BEETLE least-cost network model used to produce Somerset’s Ecological Network was developed by Forest Research (Watts et al, 2010). GIS data and mapping was produced with the support of Somerset Environmental Records Centre and First Ecology Somerset Wildlife Trust 34 Wellington Road Taunton TA1 5AW 01823 652 400 Email: [email protected] somersetwildlife.org Front Cover: Broadleaved woodland ecological network in East Mendip Contents 1. Introduction .................................................................................................................... 1 2. Policy and Legislative Background to Ecological Networks ............................................ 3 Introduction ............................................................................................................... 3 Government White Paper on the Natural Environment .............................................. 3 National Planning Policy Framework ......................................................................... 3 The Habitats and Birds Directives ............................................................................. 4 The Conservation of Habitats and Species Regulations 2010 .................................. -

Pinery Provincial Park Vascular Plant List Flowering Latin Name Common Name Community Date
Pinery Provincial Park Vascular Plant List Flowering Latin Name Common Name Community Date EQUISETACEAE HORSETAIL FAMILY Equisetum arvense L. Field Horsetail FF Equisetum fluviatile L. Water Horsetail LRB Equisetum hyemale L. ssp. affine (Engelm.) Stone Common Scouring-rush BS Equisetum laevigatum A. Braun Smooth Scouring-rush WM Equisetum variegatum Scheich. ex Fried. ssp. Small Horsetail LRB Variegatum DENNSTAEDIACEAE BRACKEN FAMILY Pteridium aquilinum (L.) Kuhn Bracken-Fern COF DRYOPTERIDACEAE TRUE FERN FAMILILY Athyrium filix-femina (L.) Roth ssp. angustum (Willd.) Northeastern Lady Fern FF Clausen Cystopteris bulbifera (L.) Bernh. Bulblet Fern FF Dryopteris carthusiana (Villars) H.P. Fuchs Spinulose Woodfern FF Matteuccia struthiopteris (L.) Tod. Ostrich Fern FF Onoclea sensibilis L. Sensitive Fern FF Polystichum acrostichoides (Michaux) Schott Christmas Fern FF ADDER’S-TONGUE- OPHIOGLOSSACEAE FERN FAMILY Botrychium virginianum (L.) Sw. Rattlesnake Fern FF FLOWERING FERN OSMUNDACEAE FAMILY Osmunda regalis L. Royal Fern WM POLYPODIACEAE POLYPODY FAMILY Polypodium virginianum L. Rock Polypody FF MAIDENHAIR FERN PTERIDACEAE FAMILY Adiantum pedatum L. ssp. pedatum Northern Maidenhair Fern FF THELYPTERIDACEAE MARSH FERN FAMILY Thelypteris palustris (Salisb.) Schott Marsh Fern WM LYCOPODIACEAE CLUB MOSS FAMILY Lycopodium lucidulum Michaux Shining Clubmoss OF Lycopodium tristachyum Pursh Ground-cedar COF SELAGINELLACEAE SPIKEMOSS FAMILY Selaginella apoda (L.) Fern. Spikemoss LRB CUPRESSACEAE CYPRESS FAMILY Juniperus communis L. Common Juniper Jun-E DS Juniperus virginiana L. Red Cedar Jun-E SD Thuja occidentalis L. White Cedar LRB PINACEAE PINE FAMILY Larix laricina (Duroi) K. Koch Tamarack Jun LRB Pinus banksiana Lambert Jack Pine COF Pinus resinosa Sol. ex Aiton Red Pine Jun-M CF Pinery Provincial Park Vascular Plant List 1 Pinery Provincial Park Vascular Plant List Flowering Latin Name Common Name Community Date Pinus strobus L. -

Application of Pontentilla Anserine, Polygonum Aviculare and Rumex Crispus Mixture Extracts in a Rabbit Model with Experimentally Induced E
animals Article Application of Pontentilla Anserine, Polygonum aviculare and Rumex Crispus Mixture Extracts in A Rabbit Model with Experimentally Induced E. coli Infection Robert Kupczy ´nski 1,* , Antoni Szumny 2 , Michał Bednarski 3, Tomasz Piasecki 3, Kinga Spitalniak-Bajerska´ 1 and Adam Roman 1 1 Department of Environment, Animal Hygiene and Welfare, Wrocław University of Environmental and Life Sciences, 51-630 Wrocław, Poland; [email protected] (K.S.-B.);´ [email protected] (A.R.) 2 Department of Chemistry, Wroclaw University of Environmental and Life Science, 50-375 Wrocław, Poland; [email protected] 3 Department of Epizootiology with Clinic of Birds and Exotic Animals, Wroclaw University of Environmental and Life Science, 50-366 Wrocław, Poland; [email protected] (M.B.); [email protected] (T.P.) * Correspondence: [email protected] Received: 27 September 2019; Accepted: 7 October 2019; Published: 9 October 2019 Simple Summary: The beneficial effect of herbs on production parameters, quality of products of animal origin, and animal health was demonstrated in numerous studies. This study aimed to determine an effect of herbal mixture on blood parameters and the anti-colibacteriosis efficacy. The rabbit model used during the experimental infection with E. coli indicates a clear effect of Rumex crispus L., Pontentilla anserine, Polygonum aviculare, whose activity involves the reduction of colonization of basically all sections of the intestines. The use of herbs in rabbits can control the activity of intestinal microbial community. Administration of a mixture of herbs to feed reduced the number of E. coli in cecum more than supplementation into water after experimental infection. -
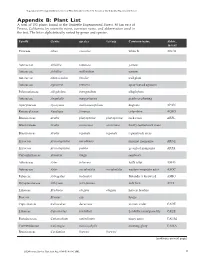
Vegetation and Ecological Characterisitics of Mixed-Conifer
Vegetation and Ecological Charactistics of Mixed-Conifer and Red Fir Forests at the Teakettle Experimental Forest Vegetation and Ecological Characteristics of Mixed-Conifer and Red Fir Forests at the Teakettle Experimental Forest Appendix B: Plant List A total of 152 plants found at the Teakettle Experimental Forest, 80 km east of Fresno, California, by scientific name, common name, and abbreviation used in the text. The list is alphabetically sorted by genus and species. Family Genus species var/ssp Common name Abbre. in text Pinaceae Abies concolor white fir ABCO Pinaceae Abies magnifica red fir ABMA Asteraceae Achillea lanulosa yarrow Asteraceae Achillea millefolium yarrow Asteraceae Adenocaulon bicolor trail plant Asteraceae Agroseris retrorsa spear-leaved agoseris Polemoniaceae Allophylum intregifolium allophylum Asteraceae Anaphalis margaritacea pearly everlasting Apocynaceae Apocynum androsaemifolium dogbane APAN Ranunculaceae Aquilegia formosa columbine AQFO Brassicaceae Arabis platysperma platysperma rock cress ARPL Brassicaceae Arabis rectissima rectissima bristly-leaved rock cress Brassicaceae Arabis repanda repanda repand rock cress Ericaceae Arctostaphylus nevadensis pinemat manzanita ARNE Ericaceae Arctostaphylus patula greenleaf manzanita ARPA Caryophyliaceae Arenaria kingii sandwort Asteraceae Aster foliaceus leafy aster ASFO Asteraceae Aster occidentalis occidentalis western mountain aster ASOC Fabaceae Astragalus bolanderi Bolander’s locoweed ASBO Dryopteridaceae Athryium felix-femina lady fern ATFI Liliaceae Brodiaea elegans elegans harvest brodeia Poaceae Bromus ssp. brome Cupressaceae Calocedrus decurrens incense cedar CADE Liliaceae Calochortus leichtlinii Leichtlin’s mariposa lily CALE Portulacaceae Calyptridium umbellatum pussy paws CAUM Convuvulaceae Calystegia malacophylla morning glory CAMA Brassicaceae Cardamine breweri breweri (continues on next page) 46 USDA Forest Service Gen.Tech. Rep. PSW-GTR-186. 2002. USDA Forest Service Gen.Tech. Rep. -

Rumex Crispus L.; Curly Dock R
A WEED REPORT from the book Weed Control in Natural Areas in the Western United States This WEED REPORT does not constitute a formal recommendation. When using herbicides always read the label, and when in doubt consult your farm advisor or county agent. This WEED REPORT is an excerpt from the book Weed Control in Natural Areas in the Western United States and is available wholesale through the UC Weed Research & Information Center (wric.ucdavis.edu) or retail through the Western Society of Weed Science (wsweedscience.org) or the California Invasive Species Council (cal-ipc.org). Rumex crispus L.; curly dock R. crispus R. obtusifolius Rumex obtusifolius L.; broadleaf dock Curly and broadleaf dock Family: Polygonaceae Range: Curly dock is found throughout the U.S., including every western state. Broadleaf dock is found in most of the western states, except Nevada, Wyoming and North Dakota. Habitat: Ditches, roadsides, wetlands, meadows, riparian areas, alfalfa and pasture fields (especially with poor drainage), orchards and other disturbed moist areas. Plants prefer wet to moist soils but tolerate periods of dryness, and can grow in most climates and soil types. Origin: R. crispus, Eurasia; R. obtusifolia, western Europe. Impact: Both species can be very competitive and outcompete more desirable vegetation for water, nutrients and light. Under certain conditions, they can accumulate soluble oxalates making them toxic to livestock. California Invasive Plant Council (Cal-IPC) Inventory: R. crispus, Limited Invasiveness Both species are erect perennials from 1.5 to 3 ft tall, occasionally to 5 ft tall. Curly dock leaves are lanceolate and up to 20 inches long, whereas broadleaf dock has lanceolate-ovate leaves that are up to 30 inches long. -

Polygonaceae of Alberta
AN ILLUSTRATED KEY TO THE POLYGONACEAE OF ALBERTA Compiled and writen by Lorna Allen & Linda Kershaw April 2019 © Linda J. Kershaw & Lorna Allen This key was compiled using informaton primarily from Moss (1983), Douglas et. al. (1999) and the Flora North America Associaton (2005). Taxonomy follows VAS- CAN (Brouillet, 2015). The main references are listed at the end of the key. Please let us know if there are ways in which the kay can be improved. The 2015 S-ranks of rare species (S1; S1S2; S2; S2S3; SU, according to ACIMS, 2015) are noted in superscript (S1;S2;SU) afer the species names. For more details go to the ACIMS web site. Similarly, exotc species are followed by a superscript X, XX if noxious and XXX if prohibited noxious (X; XX; XXX) according to the Alberta Weed Control Act (2016). POLYGONACEAE Buckwheat Family 1a Key to Genera 01a Dwarf annual plants 1-4(10) cm tall; leaves paired or nearly so; tepals 3(4); stamens (1)3(5) .............Koenigia islandica S2 01b Plants not as above; tepals 4-5; stamens 3-8 ..................................02 02a Plants large, exotic, perennial herbs spreading by creeping rootstocks; fowering stems erect, hollow, 0.5-2(3) m tall; fowers with both ♂ and ♀ parts ............................03 02b Plants smaller, native or exotic, perennial or annual herbs, with or without creeping rootstocks; fowering stems usually <1 m tall; fowers either ♂ or ♀ (unisexual) or with both ♂ and ♀ parts .......................04 3a 03a Flowering stems forming dense colonies and with distinct joints (like bamboo -

Vascular Plants and a Brief History of the Kiowa and Rita Blanca National Grasslands
United States Department of Agriculture Vascular Plants and a Brief Forest Service Rocky Mountain History of the Kiowa and Rita Research Station General Technical Report Blanca National Grasslands RMRS-GTR-233 December 2009 Donald L. Hazlett, Michael H. Schiebout, and Paulette L. Ford Hazlett, Donald L.; Schiebout, Michael H.; and Ford, Paulette L. 2009. Vascular plants and a brief history of the Kiowa and Rita Blanca National Grasslands. Gen. Tech. Rep. RMRS- GTR-233. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 44 p. Abstract Administered by the USDA Forest Service, the Kiowa and Rita Blanca National Grasslands occupy 230,000 acres of public land extending from northeastern New Mexico into the panhandles of Oklahoma and Texas. A mosaic of topographic features including canyons, plateaus, rolling grasslands and outcrops supports a diverse flora. Eight hundred twenty six (826) species of vascular plant species representing 81 plant families are known to occur on or near these public lands. This report includes a history of the area; ethnobotanical information; an introductory overview of the area including its climate, geology, vegetation, habitats, fauna, and ecological history; and a plant survey and information about the rare, poisonous, and exotic species from the area. A vascular plant checklist of 816 vascular plant taxa in the appendix includes scientific and common names, habitat types, and general distribution data for each species. This list is based on extensive plant collections and available herbarium collections. Authors Donald L. Hazlett is an ethnobotanist, Director of New World Plants and People consulting, and a research associate at the Denver Botanic Gardens, Denver, CO. -

Fallopia Japonica – Japanese Knotweed
Fallopia japonica – Japanese knotweed Japanese knotweed, sometimes referred to What is it? as donkey rhubarb for its sour red spring shoots, is a perennial plant in the Buckwheat family (Polygonaceae). It has large broad green leaves; tall, thick, sectioned and somewhat reddish zigzagging stems; and racemes of small papery flowers in summer. Photo by Liz West 2007 Other scientific names (synonyms) for Japanese knotweed are Reynoutria japonica and Polygonum cuspidatum. When does it grow? Shoots emerge from rhizomes (modified underground stems) from late March to mid-April. A spring freeze or deep frost can top kill new growth, but new shoots readily crop up from the hardy rootstalks. Growth continues rapidly once the weather begins to warm reaching heights up to 10 feet or greater by summer. R. Buczynski 2020 4.15.2020 Where is it from? Japanese knotweed is native to eastern Asia and was introduced to the United Kingdom in the 1800’s as a vigorous garden ornamental. Before becoming illegal to plant in England it was horticulturally introduced from the UK to the United States. Where is it now? Japanese knotweed has been reported extensively in the Northeastern U. S. and is currently present in all three counties (Hunterdon, Morris, and Somerset) within the upper Raritan watershed where it continues to spread into moist disturbed areas along waterways. Photo by Roger Kidd © Why is it invasive? Although knotweed can spread by seed, it is most effective at spreading underground via rhizomes that extend outward as well as downward, producing new shoots up to 70 feet away. If detached from the plant, small fragments of rhizome can survive and produce new plants wherever they land. -

Curriculum Vitae
Curriculum Vitae Alexey B. Shipunov Last revision: August 26, 2021 Contents 1 Degrees..........................................................1 2 Employment.......................................................1 3 Teaching and Mentoring Experience.........................................2 4 Awards and Funding..................................................3 5 Research Interests...................................................3 6 Research and Education Goals and Principles...................................4 7 Publications.......................................................5 8 Attended Symposia................................................... 17 9 Field Experience..................................................... 19 10 Service and Outreach.................................................. 19 11 Developing........................................................ 20 12 Professional Memberships............................................... 21 Personal Data Address Kyoto University, Univerity Museum, Yoshidahonmachi, Sakyo Ward, Kyoto, 606-8317, Japan E-mail [email protected] Fax +81 075–753–3277 Office tel. +81 075–753–3272 Web site http://ashipunov.info 1 Degrees 1998 Ph. D. (Biology), Moscow State University. Thesis title: Plantains (genera Plantago L. and Psyllium Mill., Plantaginaceae) of European Russia and adjacent territories (advisor: Dr. Vadim Tikhomirov; reviewers: Dr. Vladimir Novikov, Dr. Alexander Luferov) 1990 M. Sc. (Biology), Moscow State University. Thesis title: Knotweeds (Polygonum aviculare L. and allies)