Air Separation: Materials, Methods, Principles and Applications - an Overview D Hazel and N Gobi*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Industrial Gases and Advanced Technologies for Non-Ferrous Mining and Refining Tell Me More Experience, Understanding and Solutions
Industrial gases and advanced technologies for non-ferrous mining and refining tell me more Experience, understanding and solutions When a need for reliable gas supply or advanced technologies for your processes occurs, Air Products has the experience to help you be more successful. Working side-by-side with non-ferrous metals mining and refining facilities, we have developed in-depth knowledge and understanding of extractive and refining processes. You can leverage our experience to help increase yield, improve production, decrease harmful fugitive emissions, and reduce energy consumption and fuel cost throughout your operations. As a leading global industrial gas supplier, we offer a full range of supply modes for industrial gases, including oxygen, nitrogen, argon and hydrogen for small and large volume users, state-of-the-art equipment and a broad range of technical services based on our comprehensive global engineering experience in many critical gas applications. Our products range from packaged gases, such as portable cryogenic dewars, all the way up to energy-efficient cryogenic air separation plants and enabling equipment, such as burners and flow controls. Beyond products and services, customers count on us for over 60 years of technical knowledge and experience. As an owner and operator of over 800 air separation plants worldwide, with an understanding of current and future industry needs, we can develop and implement technical solutions that are right for your process and production challenges. It’s our goal to match your needs with a comprehensive and cost-effective industrial gas system and innovative technologies. “By engaging Air Products at an early stage in our Zambian [Kansanshi copper-gold mining] project, we were able to explore oxygen supply options and determine what we believed to be the best system for our specific process requirements. -

Liquefaction, Solidification Or Separation of Gases Or Gaseous
CPC - F25J - 2019.08 F25J LIQUEFACTION, SOLIDIFICATION OR SEPARATION OF GASES OR GASEOUS {OR LIQUEFIED GASEOUS} MIXTURES BY PRESSURE AND COLD TREATMENT {OR BY BRINGING THEM INTO THE SUPERCRITICAL STATE (cryogenic pumps F04B 37/08; gas storage vessels, gas holders F17; filing vessels with, or discharging from vessels, compressed, liquefied or solidified gases F17C; refrigeration machines, plants, or systems F25B)} Definition statement This place covers: Processes or systems for liquefying or solidifying gases or gaseous mixtures and for separating the constituents of gaseous or liquid mixtures involving the use of liquefaction or solidification by rectification or partial condensation, the processes or systems use internal and/or external refrigeration to reach very low temperatures, i.e. so-called cryogenic temperatures, in general well below -50°C; Arrangements of cold exchangers or cold accumulators in cryogenic separation or liquefaction plants. Relationships with other classification places If the principal aspect of the application concerns the liquefaction or solidification of a gaseous feed stream but comprises also purification aspects of the feed or product stream in general then the main group concerned is F25J 1/00. Similarly, if the principal aspect of the application concerns the separation of a feed stream but comprises also the withdrawal of a liquid or solid product stream then the main group concerned is F25J 3/00. If the application, however, concerns details both about liquefaction or solidification techniques as well -

Separating Air to Produce Gases
Separating air to produce gases. What is air? And what is air separation? Air is a mixture of gases, consisting primarily of nitrogen Air can be separated into its components by (78 %), oxygen (21 %) and the inert gas argon (0.9 %). means of distillation in special units. So-called air The remaining 0.1 % is made up mostly of carbon dioxide fractionating plants employ a thermal process and the inert gases neon, helium, krypton and xenon. known as cryogenic rectification to separate the individual components from one another in order to produce high-purity nitrogen, oxygen and argon in liquid and gaseous form. N2 Ar O2 Oxygen Nitrogen Argon N2 Compression of air Cooling of air Ar Separation of air Withdrawal and storage O Ambient air is drawn in, filtered and Because the gases which make 2 Separation of air into pure oxygen Gaseous oxygen and nitrogen are compressed to approx. 6 bar by a compressor. up air only liquefy at very low temperatures, and pure nitrogen is performed in two fed into pipelines for transport to the purified air in the main heat exchanger columns, the medium-pressure and the low- users, e.g. steelworks. In liquid is cooled to approx. -175 °C. The cooling pressure columns. The difference in boiling form, oxygen, nitrogen and argon Precooling of air is achieved by means of internal heat point of the constituents is exploited for are stored in tanks and transported to To separate air into its components, exchange, in which the flows of cold gas the separation process. Oxygen becomes customers by road tankers. -
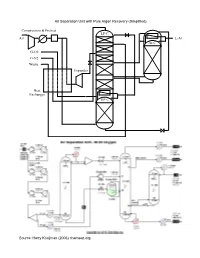
Air Separation Unit with Pure Argon Recovery (Simplified)
Air Separation Unit with Pure Argon Recovery (Simplified) Compression & Pretreat LP-C Air L-Ar Ar-C G-O2 G-N2 Waste Expander Heat Exchanger HP-C Source: Harry Kooijman (2006) chemsep.org The cryogenic air separation process is a tight integration of heat exchangers and separation columns which is completely driven by the compression of the air at the inlet of the unit. The air inlet stream is cooled and partially liquified against the leaving product streams. Nitrogen is then separated at a pressure of 6 bar in the first column and condensed against boiling oxygen at a lower pressure (around 1.2 bar). These two columns share the same column shell to minimize the temperature difference between the condensing nitrogen and evaporating oxygen. The liquid bottom product of the high pressure column is rich in oxygen and is reduced in pressure. The Joule-Thomson (JT) effect cools this rich liquid such that it can be used run the condenser of a side rectifier that separates Argon from Oxygen. This side rectifier is fed with a vapor side draw from the low pressure column. The whole process requires additional cooling which can be obtained using the JT effect in an expander, that feeds compressed air directly to the low pressure column. Thus, a certain part of the air cannot be separated but leaves the unit as a waste stream. Gaseous Nitrogen and Oxygen, and liquid Argon are the products. With temperature differences in the heat exchangers of just a few degrees Kelvin clearly there is signifficant interaction in these interconnected columns when any of the manipulated variables are adjusted or a disturbance affects one of the column controlled variables. -

Rotoflow Turboexpanders
Q&A ROTOFLOW TURBOEXPANDERS cations, they offer efficient designs for OEM with a complete offering including power recovery on new or existing pro- service in the field, spare parts inventory, cesses. Features of integrally geared types shop and field repairs, upgrades and include an expander impeller mounted rerates, performance and reliability assess- directly to the gearbox pinion to eliminate ments, remote monitoring and training. the need for high-speed coupling and an extra set of high-speed bearings. Direct Is there an expander model you’d drive and external gear approaches sim- like to highlight? plify installation and offer flexibility. Recently we have seen a lot of opportunity for our expander-compressor product line How about your energy-dissipa- designed for the hydrocarbon market. They tive expanders? offer a broad, standard set of features, Available in three types (oil-loaded, blow- while still allowing customization. With er-loaded, and resistor-loaded), Rotoflow’s development activities, we have achieved energy-dissipative turboexpanders provide a 15-25% reduction in delivery time for Darren Prosser, Global Business Manager refrigeration options for efficient hydrocar- some models. for Aftermarket at Rotoflow, discusses his bon, air separation, and liquefaction oper- company and the products and services it ations. They are often used in cryogenic What markets do you primarily offers in turbomachinery gas separation facilities. Features include serve? efficiency (87%) and a range of sizes. They Rotoflow primarily serves the industrial Tell our readers about Rotoflow have millions of successful hours of oper- gas, hydrocarbon, and energy recovery Rotoflow, an Air Products business, is a ation in Air Products plants. -

Turbo-Expanders for Cold Production and Energy Recovery
TURBO-EXPANDERS FOR COLD PRODUCTION AND ENERGY RECOVERY Everywhere in the GAS SUPPLY CHAIN Innovation for CONTINUOUS EXPANSION Cryostar – turbo expander expertise for the gas supply chain in natural gas processing and petrochemical applications For more than 30 years Cryostar has been recognized as a world leader for their superior product quality in radial inflow turbine technology, pumps, LNG boil off gas compressors and air separation. Since Cryostar began in 1966 and followed with the production of their first turbine in 1974, their focus and expertise has been to provide their customers with turbo-expander/compressor and energy recovery systems for cold production and liquid recovery with the highest efficiency ensuring economical and reliable operations. Cryostar’s head-quarters and production facility is located in Hesingue, France, just across the border from Basel, Switzerland, and employs over 400 staff in management, engineering and production. In addition, Cryostar has opened Business Centers strategically located throughout the world in the USA (Houston, California and Pennsylvania), United Kingdom, Singapore, China and Brazil to provide our customers with first class service. Today, Cryostar’s installed base, including industrial gas machines, exceeds more than 1,200 turboexpanders located in more than 60 countries worldwide. Cryostar’s innovative drive and continued commitment to invest in and develop their world class engineering skills has moved them to the forefront of technological development in all their product lines. Every turbines is designed using state-of-the-art technology to ensure each customer the very best in product design. With a policy to the commitment to excellence, Cryostar has earned a position as a world leader in their markets. -

Air Separation Plants PLANT CONSTRUCTION Solutions for Highest Purity Worldwide
SPECIALISTS IN TECHNICAL GASES MODULAR SYSTEMS Air Separation Plants PLANT CONSTRUCTION Solutions for highest purity worldwide AFTER SALES SERVICES CONTACT CRYOTEC Anlagenbau GmbH Dresdener Straße 76 04808 Wurzen Germany Phone: +49 3425 89 65 - 1610 Fax: +49 3425 89 65 - 1638 Email: [email protected] Web: www.cryotec.de For purest applications. Cryogenic air separation plants have proven themselves and been worth their value throughout the world for many years now. These systems are particularly suitable for the production of liquid and / or gaseous oxygen, nitrogen and argon. The precious products are gained from atmospheric air, based on a low-temperature rectification process. Thereby the substances are of high purity and find wide fields of application in medicine and industry. A MEMBER OF MADE IN GERMANY CRYOTEC Anlagenbau GmbH is certified per DIN EN ISO 9001 CONTACT Air Separation Plants Additional Equipment Standard and tailor-made • Pipeline service / compressors, for increasing the production pressure up to the specific requirements of the customer GASEOUS OXYGEN (GOX), NITROGEN (GN), ARGON (GAR) LIQUID OXYGEN (LOX), NITROGEN (LIN), ARGON (LAR) • Liquid gas tanks for storage of liquid products • Liquid gas pumps, vaporizers Standard and tailor-made plants of models ONG and OANG Standard and tailor-made plants of models ONL and OANL • Cylinders filling manifolds Standard models: ONG 1,000 / 2,000 / 5,000 / 10,000 Standard models: ONL 100 / 200 / 500 / 1,000 / 2,000 / 5,000 OANG 1,000 / 2,000 / 5,000 / 10,000 OANL 500 / 1,000 Specials • Automatic process control by PLC guarantees highest plant availability and safety Product Product Output* Product Purity Product Product Output* Product Purity GOX 150 - 10,000 Nm3/h 99.5 - 99.7 % by vol. -

Driving Turboexpander Technology Brochure
DRIVING EXPANDER TECHNOLOGY Atlas Copco Gas and Process Solutions Overview Driving Expander Technology Atlas Copco Gas and Process is continuously working to improve and extend the capabilities and performance of radial in-flow turbines, or turboexpanders. As a market leader for this technology, we see it as our responsibility to help our customers achieve superior productivity in their processes. To achieve this goal, we foster innovation and engage Utilizing our worldwide manufacturing and packaging in a close dialog with our customers early in the facilities, we provide the highest quality in the custom- design process. With thousands of our machines in engineered turbomachinery world. operation worldwide, we are your ideal partner to drive turboexpander technology further. Even after our machine is operating in the field, we maintain our commitment to this partnership: through the support provided by Atlas Copco’s global sales engineers, Building Productive Partnerships and the facilities and personnel of Atlas Copco service centers. These centers offer timely support for scheduled When you choose Atlas Copco turboexpanders for and emergency services, 24 hours a day. your process, you initiate a continuing partnership that extends well beyond design, delivery, and installation. From project initiation through design, delivery, and commissioning, total quality management is fundamental to every Atlas Copco project. 2 Here’s an overview of our turboexpander technology: • Custom-engineered solutions designed to fit your application • High efficiency • Robust construction • Wide operational range • Highest quality • Reliable performance • Thousands of references worldwide Exponential Expander Power Even years after the acquisition of Mafi-Trench Corporation (MTC), Atlas Copco Gas and Process is proving time and again that it is one of the world’s premier turboexpander technology companies. -

Modular Ammonia Production Plant
Modular Ammonia Production Plant Student AIChE Design Competition 2019-2020 2 TABLE OF CONTENTS ABSTRACT .................................................................................................................................................................. 3 INTRODUCTION ........................................................................................................................................................ 4 PROCESS DESCRIPTION ......................................................................................................................................... 6 PROCESS FLOW DIAGRAM AND MATERIAL BALANCE ............................................................................ 13 ENERGY BALANCE AND UTILITY REQUIREMENT ..................................................................................... 26 EQUIPMENT LIST AND UNIT DESCRIPTIONS ................................................................................................ 32 EQUIPMENT SPECIFICATION SHEETS ............................................................................................................ 35 PROCESS SAFETY CONSIDERATIONS ............................................................................................................. 36 SAFETY, HEALTH, AND ENVIRONMENTAL CONSIDERATIONS .............................................................. 40 EQUIPMENT COST SUMMARY ........................................................................................................................... 41 FIXED CAPITAL INVESTMENT -

Supplementary Material Techno-Economic Viability of Islanded Green Ammonia As a Carbon-Free Energy Vector and As a Substitute Fo
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is © The Royal Society of Chemistry 2020 Supplementary Material Techno-economic viability of islanded green ammonia as a carbon-free energy vector and as a substitute for conventional production Richard Michael Nayak-Luke and René Bañares-Alcántara Acronyms ASU Air separation unit CAGR Compound annual growth rate CAPEX Discounted capital expenditure CCGT Combined cycle gas turbine CR Geometric series common ratio FLH Full load hours equivalent GHG Greenhouse gases HB Haber-Bosch LCOA Levelised cost of ammonia LCOE Levelised cost of electricity LCOH Levelised cost of hydrogen LHV Lower heating value LNG Liquified natural gas NOLA New Orleans, Louisiana, USA OPEX Operational expenditure PV Photovoltaics RE Renewable energy SMR Steam methane reforming UK United Kingdom of Great Britain and Northern Ireland USA United States of America Nomenclature .∗ Rated power .‡ Revised value (i.e. after re-allocation of energy) $ United States Dollar 훾 Fraction of 푃퐻퐵/퐴푆푈 allocated to synthesis 휂퐿퐻푉 Efficiency relative to the lower heating value ∆퐸푡,푅푒−푎푙푙표푐푎푡푒 Potential impact on the amount of energy to re-allocate at index t for a given method 퐸푡,푀푒푡ℎ표푑 푥 Amount of energy that can be re-allocated for index t by method x H Height [m] mNH3 Discounted mass of ammonia M Molar Mass 푃퐸푙푒푐 Electrolyser power 푃퐸푙푒푐퐸푥푐푒푠푠 Excess electroliser power (i.e. power allocated for excess production of hydrogen) 푃퐻2 Hydrogen storage power 푃퐻퐵/퐴푆푈 Haber-Bosch synthesis and air separation -

Air Products Air Separation Plants Unique Technology & Unparalleled Experience
Air Products Air Separation PlantsUnique Technology & Unparalleled Experience Worldwide customers requiring large amounts of oxygen, nitrogen or argon trust Air Products’ 70 years of experience designing Column Cold Box and operating cryogenic air separation units (ASU). Whether the need is for 50 tpd or 4,000 tpd, Air Products will deliver the most cost-eective, ecient and dependable cryogenic solution from conceptual design through onstream production. 4 We have produced more than 2,000 ASU plants worldwide, and own and operate over 300. 1 Main Air Compressor (MAC) and Booster Air Compressor (BAC) 2 Adsorption Pure N2 Atmospheric air is compressed and driven through the system by a series of air compressors. While this The adsorption process removes water, carbon dioxide, nitrous oxide, and hydrocarbons from the feed image shows electrically-driven compressors, many of the larger ASUs have steam-driven compression. air stream which is needed for safe operation and high eciency. Air Products is on the leading edge of Structured Packing Matching the right compressor design for the specic ASU cycle is critical to optimize energy usage and creating the most eective absorbents in the industry, with testing facilities in China, UK, and the US. Liquid costs. Air Products engineers (machinery & process design) work directly with compressor manufacturers Because site conditions can vary, Air Products uses three dierent congurations for TSA: vertical, horizontal Nitrogen to assure the best t. Our machinery operations group is recognized as the -

Cryogenic Air Separation Plant:Layout 1.Qxd
Servomex Total Solution for Gas Analysis on Cryogenic Air Separation Plants Application Servomex provides gas analysers which have been specifically designed to meet the process control, quality assurance and safety monitoring requirements of air separation plants. Background Dry air contains approximately 78% nitrogen, 21% oxygen, and 1% argon plus low concentrations of noble gases, carbon dioxide, hydrocarbons and other impurities. Nitrogen, oxygen and argon are used by industry in large quantities and hence termed industrial gases. To support this need processes have been developed to produce these gases through the separation of air. An air separation plant divides atmospheric air into the three pure gaseous components of nitrogen, oxygen and argon. Further separation may be performed on some plants to produce other gases such as neon, krypton and xenon. Other gas components of atmospheric air, such as water vapour, carbon dioxide and hydrocarbons must be removed to ensure safety, efficient plant operation and product quality. There are different types of air separation plants but those based on cryogenic air separation are the most significant in terms of production value and volume. Cryogenic air separation presents the most opportunity for gas analysis and is now discussed in more detail. Cryogenic Air Separation Large air separation plants are based upon the cryogenic fractionation of air into its pure components. This process is based on the principle that the desired products have different boiling points and thus can be separated out from liquefied air. Due to differing user requirements there are a number of variations in the air separation cycles which are used to make industrial gas products.