Modular Ammonia Production Plant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stainless Steels in Ammonia Production
Stainless Steels in Ammonia Production Committee of Stainless Steel Producers American Iron and Steel Institute Washington, D.C. CONTENTS INTRODUCTION ...................... 4 PROCESS DESCRIPTION ....... 5 CORROSIVES IN AMMONIA PROCESSES .......... 5 CONSIDERATIONS FOR SELECTING STAINLESS STEELS .................................... 6 Desulfurization of Natural Gas ................. 6 Catalytic Steam Reforming of Natural Gas ................. 6 Carbon Monoxide Shift ......... 8 Removal of Carbon Dioxide . 10 Methanation .......................... 11 Synthesis of Ammonia ......... 11 Turbine-Driven Centrifugal Compression Trains ........ 14 STAINLESS STEELS ................ 15 Metallurgical Structure ......... 16 High-Temperature Mechanical Properties ..... 16 Thermal Conductivity ........... 20 Oxidation Resistance ........... 21 Sulfidation Resistance .......... 22 REFERENCES .......................... 22 The material presented in this booklet has been prepared for the general information of the reader. It should not be used without first securing competent advice with respect to its suitability for any given application. While the material is believed to be technically correct, neither the Committee of Stainless Steel Pro- ducers nor the companies represented on the The Committee of Stainless Steel Producers acknowledges the help Committee warrant its suitability for any gen- by Gregory Kobrin, Senior Consultant, Materials Engineering, E.I. eral or particular use. DuPont de Nemours & Company in assembling data for this booklet. single-train plants came increasing demands on materials of construc- INTRODUCTION tion. The process for making am- monia is considered to be only Approximately 12 elements are es- moderately corrosive, so considera- sential to plant growth. Of these, nit- ble use is made of carbon and low- rogen is the main nutrient and is re- alloy steels for vessels and piping. quired in much larger amounts than However, numerous applications any other element. -

Industrial Gases and Advanced Technologies for Non-Ferrous Mining and Refining Tell Me More Experience, Understanding and Solutions
Industrial gases and advanced technologies for non-ferrous mining and refining tell me more Experience, understanding and solutions When a need for reliable gas supply or advanced technologies for your processes occurs, Air Products has the experience to help you be more successful. Working side-by-side with non-ferrous metals mining and refining facilities, we have developed in-depth knowledge and understanding of extractive and refining processes. You can leverage our experience to help increase yield, improve production, decrease harmful fugitive emissions, and reduce energy consumption and fuel cost throughout your operations. As a leading global industrial gas supplier, we offer a full range of supply modes for industrial gases, including oxygen, nitrogen, argon and hydrogen for small and large volume users, state-of-the-art equipment and a broad range of technical services based on our comprehensive global engineering experience in many critical gas applications. Our products range from packaged gases, such as portable cryogenic dewars, all the way up to energy-efficient cryogenic air separation plants and enabling equipment, such as burners and flow controls. Beyond products and services, customers count on us for over 60 years of technical knowledge and experience. As an owner and operator of over 800 air separation plants worldwide, with an understanding of current and future industry needs, we can develop and implement technical solutions that are right for your process and production challenges. It’s our goal to match your needs with a comprehensive and cost-effective industrial gas system and innovative technologies. “By engaging Air Products at an early stage in our Zambian [Kansanshi copper-gold mining] project, we were able to explore oxygen supply options and determine what we believed to be the best system for our specific process requirements. -

2020 Stainless Steels in Ammonia Production
STAINLESS STEELS IN AMMONIA PRODUCTION A DESIGNERS’ HANDBOOK SERIES NO 9013 Produced by Distributed by AMERICAN IRON NICKEL AND STEEL INSTITUTE INSTITUTE STAINLESS STEELS IN AMMONIA PRODUCTION A DESIGNERS’ HANDBOOK SERIES NO 9013 Originally, this handbook was published in 1978 by the Committee of Stainless Steel Producers, American Iron and Steel Institute. The Nickel Institute republished the handbook in 2020. Despite the age of this publication the information herein is considered to be generally valid. Material presented in the handbook has been prepared for the general information of the reader and should not be used or relied on for specific applications without first securing competent advice. The Nickel Institute, the American Iron and Steel Institute, their members, staff and consultants do not represent or warrant its suitability for any general or specific use and assume no liability or responsibility of any kind in connection with the information herein. Nickel Institute [email protected] www.nickelinstitute.org CONTENTS INTRODUCTION ............................ 4 PROCESS DESCRIPTION ............ 5 CORROSIVES IN AMMONIA PROCESSES ............... 5 CONSIDERATIONS FOR SELECTING STAINLESS STEELS .......................................... 6 Desulfurization of Natural Gas ....................... 6 Catalytic Steam Reforming of Natural Gas ....................... 6 Carbon Monoxide Shift .............. 8 Removal of Carbon Dioxide . 10 Methanation ............................. 11 Synthesis of Ammonia ............. 11 -

Air Separation: Materials, Methods, Principles and Applications - an Overview D Hazel and N Gobi*
Chemical Science Review and Letters ISSN 2278-6783 Research Article Air Separation: Materials, Methods, Principles and Applications - An Overview D Hazel and N Gobi* Department of Textile Technology, Anna University, Chennai – 600025, Tamil Nadu, India Abstract Air separation process is an emerging technology which is widely used in Keywords: Air separation, methods, many fields. The air separation methods, processing parameters, techniques principles, membrane, applications and external factors such as pressure and temperature can be varied based on the end use application. Inert gas generation, oxygen enrichment, natural *Correspondence gas dehydration, sour gas treatment, air humidification, purging gas, acid Author: N Gobi gas treatment etc., are the main application area of the air separation. Now a Email: [email protected] days, binary gas separation widely used in huge variety of application. It also plays a role in reduction of air pollutants in many industries. Recently membrane takes a huge part in air separation as efficient and simple method. The usage of membrane will reduce the cost and energy. This paper reviews the available methods, principle, properties and applications of different air separation process and also it reviews about the different forms of available materials for air separation. Introduction Recently, air separation has an application in many fields due to its requirements in the present scenario. Air separation is the process of segregating primary components from the atmospheric air. Air separation in other words reveals the information of selective separation of air components from its volume and oxygen plays an important role in chemical industry, medical field, combustion process [1], isolation of pollutants and recovery of reactants [2]. -

The Haber Process
Making ammonia - the Haber process Background During the last century, the populations of Europe and America rose very rapidly. More food and more crops were needed to feed more and more people. So farmers began to use nitrogen compounds as fertilisers. The main source of nitrogen compounds for fertilisers was sodium nitrate from Chile. By 1900 supplies of this were running out. Another supply of nitrogen had to be found or many people would starve. The obvious source of nitrogen was the air (about 78% of the air is nitrogen). Unfortunately, nitrogen is not very reactive. This made it difficult to convert it into ammonium salts and nitrates for use as fertilisers. A German chemist called Fritz Haber solved the problem. In 1904, Haber began studying the reaction between nitrogen and hydrogen. By 1908 he had found the conditions needed to make ammonia (NH3). Eventually, the Haber process became the most important method of manufacturing ammonia. 1. Why did farmers start to use nitrogen compounds as fertilisers? 2. What problem did farmers face in 1900? 3. How long did it take Fritz Haber to work out the conditions needed to make nitrogen and hydrogen react together? 4. What does the Haber process make? 5. Haber was an apprentice plumber before studying to become a chemist. How was Haber’s background useful to him as a chemist? The Haber process The raw materials for the Haber process are Natural gas, air and water. In the first stage, Natural gas (which is mostly methane) is reacted with steam to produce carbon dioxide and hydrogen. -

Nitrogenase; Nitrogen Fixation Vs Haber-Bosch Process
Nitrogenase; Nitrogen Fixation vs Haber-Bosch Process Anna Balinski, Jacob Watson Texas A&M University, College Station, TX 77843 Abstract Comparison of Processes Haber-Bosch Process Overview:The Nitrogen Cycle is a chemical cycle which recycles nitrogen into usable forms, such as ammonium, nitrates, and nitrites. Nitrogen and nitrogen compounds are important Haber-Bosch Reaction because they make up our atmosphere as well as our earthly environments. Nitrogen can be recycled into ammonium naturally via nitrogen fixing bacteria, or synthetically using the + - Discovered by Fritz Haber, process scaled up by Haber-Bosch process. Nitrogen fixation in bacteria follows the reaction N2 + 8 H + 8 e 2 • Carl Bosch NH3 + H2 and is powered by the hydrolysis of 16 ATP equivalents. The Haber-Bosch process Response to Germany’s need for ammonia for follows the reaction scheme of N2 + 3 H2 2 NH3 and is powered by using high pressures, • temperatures, and catalysts. Using the Haber-Bosch process an optimum yield of 97% explosive during WWI ammonium can be obtained. These reactions both use diatomic nitrogen as well as Nitrogen Fixation Reaction • Prolonged WWI when Germany was able to hydrogen, but differ in their final products as bacterial nitrogen fixation releases hydrogen produce fertilizer and explosives gas as a byproduct. Bacteria in the soil use nitrogen to create energy to grow and reproduce Fritz Haber Carl Bosch as well as to introduce nitrogen for use by other species. The Haber-Bosch process produces ammonium which can be used for a range of activities such as the production of fertilizers, or even explosives. -

Liquefaction, Solidification Or Separation of Gases Or Gaseous
CPC - F25J - 2019.08 F25J LIQUEFACTION, SOLIDIFICATION OR SEPARATION OF GASES OR GASEOUS {OR LIQUEFIED GASEOUS} MIXTURES BY PRESSURE AND COLD TREATMENT {OR BY BRINGING THEM INTO THE SUPERCRITICAL STATE (cryogenic pumps F04B 37/08; gas storage vessels, gas holders F17; filing vessels with, or discharging from vessels, compressed, liquefied or solidified gases F17C; refrigeration machines, plants, or systems F25B)} Definition statement This place covers: Processes or systems for liquefying or solidifying gases or gaseous mixtures and for separating the constituents of gaseous or liquid mixtures involving the use of liquefaction or solidification by rectification or partial condensation, the processes or systems use internal and/or external refrigeration to reach very low temperatures, i.e. so-called cryogenic temperatures, in general well below -50°C; Arrangements of cold exchangers or cold accumulators in cryogenic separation or liquefaction plants. Relationships with other classification places If the principal aspect of the application concerns the liquefaction or solidification of a gaseous feed stream but comprises also purification aspects of the feed or product stream in general then the main group concerned is F25J 1/00. Similarly, if the principal aspect of the application concerns the separation of a feed stream but comprises also the withdrawal of a liquid or solid product stream then the main group concerned is F25J 3/00. If the application, however, concerns details both about liquefaction or solidification techniques as well -

Separating Air to Produce Gases
Separating air to produce gases. What is air? And what is air separation? Air is a mixture of gases, consisting primarily of nitrogen Air can be separated into its components by (78 %), oxygen (21 %) and the inert gas argon (0.9 %). means of distillation in special units. So-called air The remaining 0.1 % is made up mostly of carbon dioxide fractionating plants employ a thermal process and the inert gases neon, helium, krypton and xenon. known as cryogenic rectification to separate the individual components from one another in order to produce high-purity nitrogen, oxygen and argon in liquid and gaseous form. N2 Ar O2 Oxygen Nitrogen Argon N2 Compression of air Cooling of air Ar Separation of air Withdrawal and storage O Ambient air is drawn in, filtered and Because the gases which make 2 Separation of air into pure oxygen Gaseous oxygen and nitrogen are compressed to approx. 6 bar by a compressor. up air only liquefy at very low temperatures, and pure nitrogen is performed in two fed into pipelines for transport to the purified air in the main heat exchanger columns, the medium-pressure and the low- users, e.g. steelworks. In liquid is cooled to approx. -175 °C. The cooling pressure columns. The difference in boiling form, oxygen, nitrogen and argon Precooling of air is achieved by means of internal heat point of the constituents is exploited for are stored in tanks and transported to To separate air into its components, exchange, in which the flows of cold gas the separation process. Oxygen becomes customers by road tankers. -

Haber Process - Wikipedia 7/17/20, 9�20 AM
Haber process - Wikipedia 7/17/20, 9)20 AM Haber process The Haber process,[1] also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today.[2][3] It is named after its inventors, the German chemists Fritz Haber and Carl Bosch, who developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using a metal catalyst under high temperatures and pressures: Before the development of the Haber process, ammonia had been difficult to produce on an industrial scale,[4][5][6] with early methods such as the Birkeland–Eyde process and Frank– Caro process all being highly inefficient. Although the Haber process is mainly used to produce fertilizer today, during World War I it provided Germany with a source of ammonia for the production of explosives, compensating for the Allied Powers' trade blockade on Chilean saltpeter. Fritz Haber, 1918 Contents History Process Sources of hydrogen Reaction rate and equilibrium Catalysts Production of iron-based catalysts Catalysts other than iron Second generation catalysts Catalyst poisons Industrial production Synthesis parameters Large-scale technical implementation Mechanism Elementary steps Energy diagram Economic and environmental aspects See also References External links History Throughout the 19th century the demand for nitrates and ammonia for use as fertilizers and industrial feedstocks had been steadily increasing. The main source was mining niter deposits. At the beginning of the 20th century it was being predicted that these reserves could not satisfy future demands,[7] and research into new potential sources of ammonia became more important. -
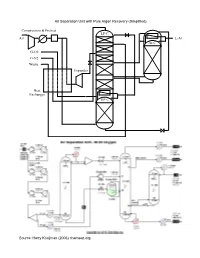
Air Separation Unit with Pure Argon Recovery (Simplified)
Air Separation Unit with Pure Argon Recovery (Simplified) Compression & Pretreat LP-C Air L-Ar Ar-C G-O2 G-N2 Waste Expander Heat Exchanger HP-C Source: Harry Kooijman (2006) chemsep.org The cryogenic air separation process is a tight integration of heat exchangers and separation columns which is completely driven by the compression of the air at the inlet of the unit. The air inlet stream is cooled and partially liquified against the leaving product streams. Nitrogen is then separated at a pressure of 6 bar in the first column and condensed against boiling oxygen at a lower pressure (around 1.2 bar). These two columns share the same column shell to minimize the temperature difference between the condensing nitrogen and evaporating oxygen. The liquid bottom product of the high pressure column is rich in oxygen and is reduced in pressure. The Joule-Thomson (JT) effect cools this rich liquid such that it can be used run the condenser of a side rectifier that separates Argon from Oxygen. This side rectifier is fed with a vapor side draw from the low pressure column. The whole process requires additional cooling which can be obtained using the JT effect in an expander, that feeds compressed air directly to the low pressure column. Thus, a certain part of the air cannot be separated but leaves the unit as a waste stream. Gaseous Nitrogen and Oxygen, and liquid Argon are the products. With temperature differences in the heat exchangers of just a few degrees Kelvin clearly there is signifficant interaction in these interconnected columns when any of the manipulated variables are adjusted or a disturbance affects one of the column controlled variables. -

The Haber Process
The Haber process MAGS Hey. I’m Mags and this is Cal. Cal. CAL Hey, you can’t hurry a good reaction. This episode Mags has made a video about ammonia and the Haber process. I need to get ready for my hot date. MAGS I think you need a ‘Cal process’ to make you ready. Here’s my video. Comb your hair. CAL I have. MAGS You’d find ammonia in my hair, because it’s in hair dye, and also fertilisers, cleaning fluids, even explosives. So how’s ammonia produced, I hear you ask. Molecules of nitrogen, which comes from the air, and hydrogen, are mixed in a reaction called the Haber process. And it’s a reversible reaction, so the arrows go in both directions. Our Auntie Trish works at a factory making ammonia for fertilisers. It’s a growth industry. Because fertiliser grows things. Check it out… Wow, this looks expensive. TRISH Yup, this factory spends a lot of money, especially on the energy bill. The energy cost goes up if we use a higher temperature. Environmental factors also need to be considered. The two main factors affecting the chemical reaction here are temperature, and pressure. MAGS So, heat up the nitrogen and hydrogen, increase the pressure, then… TRISH Molecules move faster, and collide more, then it’s ‘ammonia o’clock’. MAGS Is that an actual time? bbc.co.uk/bitesize © Copyright BBC TRISH No. We also speed up the reaction with a catalyst – with the Haber process, that’s iron. We need to replace the iron over time as it gets poisoned. -

Rotoflow Turboexpanders
Q&A ROTOFLOW TURBOEXPANDERS cations, they offer efficient designs for OEM with a complete offering including power recovery on new or existing pro- service in the field, spare parts inventory, cesses. Features of integrally geared types shop and field repairs, upgrades and include an expander impeller mounted rerates, performance and reliability assess- directly to the gearbox pinion to eliminate ments, remote monitoring and training. the need for high-speed coupling and an extra set of high-speed bearings. Direct Is there an expander model you’d drive and external gear approaches sim- like to highlight? plify installation and offer flexibility. Recently we have seen a lot of opportunity for our expander-compressor product line How about your energy-dissipa- designed for the hydrocarbon market. They tive expanders? offer a broad, standard set of features, Available in three types (oil-loaded, blow- while still allowing customization. With er-loaded, and resistor-loaded), Rotoflow’s development activities, we have achieved energy-dissipative turboexpanders provide a 15-25% reduction in delivery time for Darren Prosser, Global Business Manager refrigeration options for efficient hydrocar- some models. for Aftermarket at Rotoflow, discusses his bon, air separation, and liquefaction oper- company and the products and services it ations. They are often used in cryogenic What markets do you primarily offers in turbomachinery gas separation facilities. Features include serve? efficiency (87%) and a range of sizes. They Rotoflow primarily serves the industrial Tell our readers about Rotoflow have millions of successful hours of oper- gas, hydrocarbon, and energy recovery Rotoflow, an Air Products business, is a ation in Air Products plants.