Introduction to Ammonia Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Industrial Gases and Advanced Technologies for Non-Ferrous Mining and Refining Tell Me More Experience, Understanding and Solutions
Industrial gases and advanced technologies for non-ferrous mining and refining tell me more Experience, understanding and solutions When a need for reliable gas supply or advanced technologies for your processes occurs, Air Products has the experience to help you be more successful. Working side-by-side with non-ferrous metals mining and refining facilities, we have developed in-depth knowledge and understanding of extractive and refining processes. You can leverage our experience to help increase yield, improve production, decrease harmful fugitive emissions, and reduce energy consumption and fuel cost throughout your operations. As a leading global industrial gas supplier, we offer a full range of supply modes for industrial gases, including oxygen, nitrogen, argon and hydrogen for small and large volume users, state-of-the-art equipment and a broad range of technical services based on our comprehensive global engineering experience in many critical gas applications. Our products range from packaged gases, such as portable cryogenic dewars, all the way up to energy-efficient cryogenic air separation plants and enabling equipment, such as burners and flow controls. Beyond products and services, customers count on us for over 60 years of technical knowledge and experience. As an owner and operator of over 800 air separation plants worldwide, with an understanding of current and future industry needs, we can develop and implement technical solutions that are right for your process and production challenges. It’s our goal to match your needs with a comprehensive and cost-effective industrial gas system and innovative technologies. “By engaging Air Products at an early stage in our Zambian [Kansanshi copper-gold mining] project, we were able to explore oxygen supply options and determine what we believed to be the best system for our specific process requirements. -

Impact of Rising Natural Gas Prices on U.S. Ammonia Supply
A Report from the Economic Research Service United States Department www.ers.usda.gov of Agriculture WRS-0702 August 2007 Impact of Rising Natural Gas Prices on U.S. Ammonia Supply Wen-yuan Huang Abstract The volatile and upward trend in U.S. natural gas prices from 2000-06 has led to a 17- percent decline in the Nation’s annual aggregate supply of ammonia. During the period, U.S. ammonia production declined 44 percent, while U.S. ammonia imports increased Contents 115 percent. Also, the share of U.S.-produced ammonia in the U.S. aggregate supply of ammonia dropped from 80 to 55 percent, while the share from imports increased from Introduction ........................1 15 percent to 42 percent. Meanwhile, ammonia prices paid by farmers increased from $227 per ton in 2000 to $521 per ton in 2006, an increase of 130 percent. Natural gas is Background ........................3 the main input used to produce ammonia. Additional increases in U.S. natural gas prices could lead to a further decline in domestic ammonia production and an even greater rise Impact of Natural Gas Prices on Ammonia in ammonia imports. Prices ................................5 Keywords: Natural gas and ammonia prices, ammonia supply, nitrogen fertilizers Effects of Natural Gas Prices on Ammonia Producers .........................6 Acknowledgments Effects of Rising Natural Gas Prices on Farmers .....................10 The author thanks the Fertilizer Institute, the International Center for Soil Fertility and Future Sources of Agricultural Development, and the National Gas Company of Trinidad and Tobago Ammonia Supply in Limited for providing data used in this study. The author also thanks the following indi- the United States ............12 viduals for their helpful suggestions: Stan Daberkow and Greg Pompelli of USDA’s Economic Research Service and James Duffield of USDA’s Office of Energy Policy Summary and Implications ......................15 and New Uses, and Harry Vroomen, the Fertilizer Institute. -

2020 Stainless Steels in Ammonia Production
STAINLESS STEELS IN AMMONIA PRODUCTION A DESIGNERS’ HANDBOOK SERIES NO 9013 Produced by Distributed by AMERICAN IRON NICKEL AND STEEL INSTITUTE INSTITUTE STAINLESS STEELS IN AMMONIA PRODUCTION A DESIGNERS’ HANDBOOK SERIES NO 9013 Originally, this handbook was published in 1978 by the Committee of Stainless Steel Producers, American Iron and Steel Institute. The Nickel Institute republished the handbook in 2020. Despite the age of this publication the information herein is considered to be generally valid. Material presented in the handbook has been prepared for the general information of the reader and should not be used or relied on for specific applications without first securing competent advice. The Nickel Institute, the American Iron and Steel Institute, their members, staff and consultants do not represent or warrant its suitability for any general or specific use and assume no liability or responsibility of any kind in connection with the information herein. Nickel Institute [email protected] www.nickelinstitute.org CONTENTS INTRODUCTION ............................ 4 PROCESS DESCRIPTION ............ 5 CORROSIVES IN AMMONIA PROCESSES ............... 5 CONSIDERATIONS FOR SELECTING STAINLESS STEELS .......................................... 6 Desulfurization of Natural Gas ....................... 6 Catalytic Steam Reforming of Natural Gas ....................... 6 Carbon Monoxide Shift .............. 8 Removal of Carbon Dioxide . 10 Methanation ............................. 11 Synthesis of Ammonia ............. 11 -

Air Separation: Materials, Methods, Principles and Applications - an Overview D Hazel and N Gobi*
Chemical Science Review and Letters ISSN 2278-6783 Research Article Air Separation: Materials, Methods, Principles and Applications - An Overview D Hazel and N Gobi* Department of Textile Technology, Anna University, Chennai – 600025, Tamil Nadu, India Abstract Air separation process is an emerging technology which is widely used in Keywords: Air separation, methods, many fields. The air separation methods, processing parameters, techniques principles, membrane, applications and external factors such as pressure and temperature can be varied based on the end use application. Inert gas generation, oxygen enrichment, natural *Correspondence gas dehydration, sour gas treatment, air humidification, purging gas, acid Author: N Gobi gas treatment etc., are the main application area of the air separation. Now a Email: [email protected] days, binary gas separation widely used in huge variety of application. It also plays a role in reduction of air pollutants in many industries. Recently membrane takes a huge part in air separation as efficient and simple method. The usage of membrane will reduce the cost and energy. This paper reviews the available methods, principle, properties and applications of different air separation process and also it reviews about the different forms of available materials for air separation. Introduction Recently, air separation has an application in many fields due to its requirements in the present scenario. Air separation is the process of segregating primary components from the atmospheric air. Air separation in other words reveals the information of selective separation of air components from its volume and oxygen plays an important role in chemical industry, medical field, combustion process [1], isolation of pollutants and recovery of reactants [2]. -

Wind to Hydrogen to Ammonia Pilot Project
Renewable Energy Storage and Utilization Using Hydrogen and Anhydrous Ammonia MN House Energy and Climate Committee August 27, 2020 Michael Reese, Director - Renewable Energy Dr. Prodromos Daoutidis, Professor UMN West Central Research and Outreach Center UMN Dept. of Chemical Engineering and Morris, MN Material Science Minneapolis, MN Renewable Ammonia for Energy Storage University of Minnesota is a global research leader in this field. NH3 UMM 1.65 MW 131 kW solar PV Wind Turbine and two 10 kW small wind turbines 1.65 MW Vestas V82 Wind Turbine H2 & NH3 Pilot Plant Existing Wind-to-Ammonia Pilot Plant at the UMN WCROC, Morris, MN G. Soloveichik, US Dept. of Energy, 2016 Slide 2 Benefits for using ammonia as regional-scale energy storage •Provides both distribution and transmission-scale energy storage, •Wide range of fuel uses (ICE genset, turbine, fuel cell, and thermal energy) •Seasonal storage capability, •Grid stabilization, •Readily dispatchable generation capacity (Peak load and peak fertilizer months are complementary. High N fertilizer demand is during utility shoulder seasons.), •Enables utilization of excess generation of wind, solar, and nuclear (low and negatively priced power within the Regional Transmission Organization / Independent System Operator), •Provides emergency backup outside of traditional energy sources •Flexibility between renewable and non-renewable generation (allows choice of carbon intensity of fuel between green and brown ammonia) •Significant levels of ammonia storage already in the Midwest (and usually -

Process Intensification with Integrated Water-Gas-Shift Membrane Reactor Reactor Concept (Left)
INDUSTRIAL TECHNOLOGIES PROGRAM Process Intensification with Integrated Water-Gas-Shift Membrane Reactor Reactor concept (left). Flow diagram (middle): Hydrogen (H2) permeates the membrane where nitrogen (N2) sweeps the gas to Hydrogen-Selective Membranes for High- produce a high-pressure H2/N2 gas stream. Membrane diagram Pressure Hydrogen Separation (right): The H2-selective membrane allows the continuous removal of the H2 produced in the water-gas-shift (WGS) reaction. This allows for the near-complete conversion of carbon monoxide (CO) This project will develop hydrogen-selective membranes for an innovative water-gas-shift reactor that improves gas separation to carbon dioxide (CO2) and for the separation of H2 from CO2. efficiency, enabling reduced energy use and greenhouse gas Image Courtesy of General Electric Company, Western Research Institute, emissions. and Idaho National Laboratory. Benefits for Our Industry and Our Nation Introduction The development of an integrated WGS-MR for hydrogen The goal of process intensification is to reduce the equipment purification and carbon capture will result in fuel flexibility footprint, energy consumption, and environmental impact of as well as environmental, energy, and economic benefits. manufacturing processes. Commercialization of this technology has the potential to achieve the following: One candidate for process intensification is gasification, a common method by which hydrocarbon feedstocks such as coal, • A reduction in energy use during the separation process by biomass, and organic waste are reacted with a controlled amount replacing a conventional WGS reactor and CO2 removal system of oxygen and steam to produce synthesis gas (syngas), which with a WGS-MR and downsized CO2 removal unit is composed primarily of hydrogen (H2) and carbon monoxide (CO). -

Liquefaction, Solidification Or Separation of Gases Or Gaseous
CPC - F25J - 2019.08 F25J LIQUEFACTION, SOLIDIFICATION OR SEPARATION OF GASES OR GASEOUS {OR LIQUEFIED GASEOUS} MIXTURES BY PRESSURE AND COLD TREATMENT {OR BY BRINGING THEM INTO THE SUPERCRITICAL STATE (cryogenic pumps F04B 37/08; gas storage vessels, gas holders F17; filing vessels with, or discharging from vessels, compressed, liquefied or solidified gases F17C; refrigeration machines, plants, or systems F25B)} Definition statement This place covers: Processes or systems for liquefying or solidifying gases or gaseous mixtures and for separating the constituents of gaseous or liquid mixtures involving the use of liquefaction or solidification by rectification or partial condensation, the processes or systems use internal and/or external refrigeration to reach very low temperatures, i.e. so-called cryogenic temperatures, in general well below -50°C; Arrangements of cold exchangers or cold accumulators in cryogenic separation or liquefaction plants. Relationships with other classification places If the principal aspect of the application concerns the liquefaction or solidification of a gaseous feed stream but comprises also purification aspects of the feed or product stream in general then the main group concerned is F25J 1/00. Similarly, if the principal aspect of the application concerns the separation of a feed stream but comprises also the withdrawal of a liquid or solid product stream then the main group concerned is F25J 3/00. If the application, however, concerns details both about liquefaction or solidification techniques as well -

Reducing CO2 Slip from the Syngas Unit of an Ammonia Plant
Reducing CO2 Slip from the Syngas Unit of an Ammonia Plant Eddy Cooper Agrium Carseland Nitrogen Operations, PO Box 5370, Station A, Calgary, Alberta, Canada T2H 2P4 Ralph Weiland Optimized Gas Treating, Inc., 12337 Jones Rd., Suite 432, Houston, TX 77070 The Agrium Carseland Nitrogen plant near Carseland, Alberta uses a promoted MDEA solvent to remove CO2 from ammonia synthesis gas. The amine absorber in this unit was found to be operating unexpectedly close to its treating capacity limit, and treating performance was overly sensitive to production rate. A study was undertaken to determine the cause. Pushing plant throughput had an exponentially deleterious effect on absorber performance. The relationship between CO2 slip and production rate predicted by the ProTreat mass transfer rate-based simulator was vastly different from plant measurements, indicating that performance was not what it should be. ProTreat also predicted a temperature bulge close to the base of the column. However, a thermal scan of the absorber showed the temperature bulge was actually much further up the column on the same side of the tower as the vapour inlet, and that it was relatively flat. The asymmetry of temperature is a good indication of flow maldistribution. The thermal scan indicated poor absorption performance across the bottom 5 metres of packing; whereas, absorption rates (and therefore temperatures) should have been highest there. Channeling in the bottom of the absorber was identified to be the cause of the problem. One possible cause of channeling was the lack of liquid redistribution anywhere in the 13-m deep packed bed (a liquid redistributor at least every 6 metres is recommended at a minimum). -

Isoboost® System Save Energy with Isoboost Isoboost Overview
IsoBoost® System Save Energy with IsoBoost IsoBoost Overview Energy Recovery’s IsoBoost is a hydraulic system that recovers pressure energy and increases the reliability of pumping systems. In ammonia production, IsoBoost is used in CO2 removal, while in gas processing, the system is used in acid gas removal. It helps plants save energy, reduce maintenance, and run more profitably. The core of IsoBoost is a proprietary liquid-to-liquid turbocharger. With three times the reliability of a traditional pump, the turbocharger recovers energy from the letdown of a high-pressure fluid and transfers it to a low-pressure fluid to reduce the energy required for pumping. By replacing a complex pump and motor system with the simple and efficient IsoBoost, plants can expect millions of dollars in energy savings and a big drop in maintenance over the life of a plant. With IsoBoost, plants save up to 50% of electric power costs. Plants can save up to 50% of total electric power consumption for the acid gas removal circuit. 2 Energy Recovery IsoBoost System ISOBOOST BENEFITS IsoBoost Delivers Multiple Benefits to Plants Estimated Savings with IsoBoost Save Energy Amine System Economics IsoBoost will save millions of dollars in energy over Electric Power 50% 80% the life of a plant. Plants can save up to 50% of Maintenance Savings 50% 20% total electric power consumption for the acid gas in CO2 Removal Unit removal circuit, and the system will perform at up to 80% efficiency. Improve Reliability, Availability, Maintainability IsoBoost is up to three times as reliable as a pump. Compare its mean time to failure (MTTF) of 10 years to the 2.9 years MTTF of a traditional multistage, centrifugal motor driven pump. -

Separating Air to Produce Gases
Separating air to produce gases. What is air? And what is air separation? Air is a mixture of gases, consisting primarily of nitrogen Air can be separated into its components by (78 %), oxygen (21 %) and the inert gas argon (0.9 %). means of distillation in special units. So-called air The remaining 0.1 % is made up mostly of carbon dioxide fractionating plants employ a thermal process and the inert gases neon, helium, krypton and xenon. known as cryogenic rectification to separate the individual components from one another in order to produce high-purity nitrogen, oxygen and argon in liquid and gaseous form. N2 Ar O2 Oxygen Nitrogen Argon N2 Compression of air Cooling of air Ar Separation of air Withdrawal and storage O Ambient air is drawn in, filtered and Because the gases which make 2 Separation of air into pure oxygen Gaseous oxygen and nitrogen are compressed to approx. 6 bar by a compressor. up air only liquefy at very low temperatures, and pure nitrogen is performed in two fed into pipelines for transport to the purified air in the main heat exchanger columns, the medium-pressure and the low- users, e.g. steelworks. In liquid is cooled to approx. -175 °C. The cooling pressure columns. The difference in boiling form, oxygen, nitrogen and argon Precooling of air is achieved by means of internal heat point of the constituents is exploited for are stored in tanks and transported to To separate air into its components, exchange, in which the flows of cold gas the separation process. Oxygen becomes customers by road tankers. -
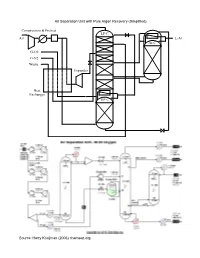
Air Separation Unit with Pure Argon Recovery (Simplified)
Air Separation Unit with Pure Argon Recovery (Simplified) Compression & Pretreat LP-C Air L-Ar Ar-C G-O2 G-N2 Waste Expander Heat Exchanger HP-C Source: Harry Kooijman (2006) chemsep.org The cryogenic air separation process is a tight integration of heat exchangers and separation columns which is completely driven by the compression of the air at the inlet of the unit. The air inlet stream is cooled and partially liquified against the leaving product streams. Nitrogen is then separated at a pressure of 6 bar in the first column and condensed against boiling oxygen at a lower pressure (around 1.2 bar). These two columns share the same column shell to minimize the temperature difference between the condensing nitrogen and evaporating oxygen. The liquid bottom product of the high pressure column is rich in oxygen and is reduced in pressure. The Joule-Thomson (JT) effect cools this rich liquid such that it can be used run the condenser of a side rectifier that separates Argon from Oxygen. This side rectifier is fed with a vapor side draw from the low pressure column. The whole process requires additional cooling which can be obtained using the JT effect in an expander, that feeds compressed air directly to the low pressure column. Thus, a certain part of the air cannot be separated but leaves the unit as a waste stream. Gaseous Nitrogen and Oxygen, and liquid Argon are the products. With temperature differences in the heat exchangers of just a few degrees Kelvin clearly there is signifficant interaction in these interconnected columns when any of the manipulated variables are adjusted or a disturbance affects one of the column controlled variables. -

Natural Gas to Ammonia As a Potential Solution for British Columbia
SPECIFIC REPORT Natural Gas to Ammonia as a Potential Solution for British Columbia University of Ontario Institute of Technology Prof. Dr. Ibrahim Dincer Yusuf Bicer Hydrofuel Inc. Greg Vezina Chairman and CEO August 9, 2016 1 Abstract Ammonia is projected to be a potential energy solution with high hydrogen content in the near future. In recent years, expectations are rising for hydrogen and ammonia as a medium for storage and transportation of energy in the mass introduction and use of renewable energy. Both storage and transport of hydrogen are considered an important issue since hydrogen is a gas under normal temperature and pressure. Hydrogen carriers are mediums that convert hydrogen into chemical substances containing large amounts of hydrogen, to simplify storage and transport processes. Hydrogen carriers include ammonia synthesized from nitrogen and hydrogen that can be used for direct combustion. Ammonia becomes an important energy carrier that does not contain any carbon atoms and has a high hydrogen ratio. Therefore, it is evaluated as a power- generating fuel. Since ammonia produces mainly water and nitrogen on combustion, replacing a part of conventional fuel with ammonia will have a large effect in reducing carbon dioxide emissions. Ammonia as a sustainable fuel can be used in all types of combustion engines, gas turbines, burners with only small modifications and directly in fuel cells which is a very important advantage compared to other type of fuels. In an ammonia economy, the availability of a pipeline to the residential area could supply ammonia to fuel cells, stationary generators, furnaces/boilers and vehicles which will bring a non-centralized power generation and enable a greener world.