A Developmental Perspective on the Origin of Morphological Disparity in Domesticated Horses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FEI Regulations for Equestrian Events at the Olympic Games
FEI Fédération Equestre Internationale FEI Regulations for Equestrian Events at the Olympic Games 24th Edition, Effective for the Olympic Games Tokyo 2020 23 July-8 August 2021 Fédération Equestre Internationale t +41 21 310 47 47 HM King Hussein I Building f +41 21 310 47 60 Chemin de la Joliette 8 www.fei.org 1006 Lausanne Switzerland Printed in Switzerland Copyright © 2018 Fédération Equestre Internationale 7 December 2018 Updated on 21 December 2018 Updated on 30 December 2018 Updated on 18 April 2019 Updated on 3 October 2019 Updated on 24 June 2020 Updated on 16 June 2021 FEI Regulations for Equestrian Events Tokyo (JPN) 2020 Olympic Games TABLE OF CONTENTS THE FEI CODE OF CONDUCT FOR THE WELFARE OF THE HORSE .................................. 4 CHAPTER I GENERAL .................................................................................................. 6 ARTICLE 600 – INTRODUCTION .................................................................................................. 6 ARTICLE 601 –COMPETITIONS .................................................................................................... 6 ARTICLE 602 – COMPETITION SCHEDULE .................................................................................... 7 ARTICLE 603 – CLASSIFICATION, MEDALS & PRIZES..................................................................... 7 ARTICLE 604 – QUOTA .............................................................................................................. 8 ARTICLE 605 - AP ALTERNATE ATHLETES, RESERVE HORSES, -

Population Genetic Analysis of the Estonian Native Horse Suggests Diverse and Distinct Genetics, Ancient Origin and Contribution from Unique Patrilines
G C A T T A C G G C A T genes Article Population Genetic Analysis of the Estonian Native Horse Suggests Diverse and Distinct Genetics, Ancient Origin and Contribution from Unique Patrilines Caitlin Castaneda 1 , Rytis Juras 1, Anas Khanshour 2, Ingrid Randlaht 3, Barbara Wallner 4, Doris Rigler 4, Gabriella Lindgren 5,6 , Terje Raudsepp 1,* and E. Gus Cothran 1,* 1 College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX 77843, USA 2 Sarah M. and Charles E. Seay Center for Musculoskeletal Research, Texas Scottish Rite Hospital for Children, Dallas, TX 75219, USA 3 Estonian Native Horse Conservation Society, 93814 Kuressaare, Saaremaa, Estonia 4 Institute of Animal Breeding and Genetics, University of Veterinary Medicine Vienna, 1210 Vienna, Austria 5 Department of Animal Breeding and Genetics, Swedish University of Agricultural Sciences, 75007 Uppsala, Sweden 6 Livestock Genetics, Department of Biosystems, KU Leuven, B-3001 Leuven, Belgium * Correspondence: [email protected] (T.R.); [email protected] (E.G.C.) Received: 9 August 2019; Accepted: 13 August 2019; Published: 20 August 2019 Abstract: The Estonian Native Horse (ENH) is a medium-size pony found mainly in the western islands of Estonia and is well-adapted to the harsh northern climate and poor pastures. The ancestry of the ENH is debated, including alleged claims about direct descendance from the extinct Tarpan. Here we conducted a detailed analysis of the genetic makeup and relationships of the ENH based on the genotypes of 15 autosomal short tandem repeats (STRs), 18 Y chromosomal single nucleotide polymorphisms (SNPs), mitochondrial D-loop sequence and lateral gait allele in DMRT3. -

Camels, Donkeys and Caravan Trade: an Emerging Context from Baraqish
Camels, donkeys and caravan trade: an emerging context from Baraqish,- ancient Yathill (Wadi- - al-Jawf, Yemen) Francesco G. FEDELE Laboratorio di Antropologia, Università di Napoli ‘Federico II’, Naples, Italy (retired), current address: via Foligno 78/10, 10149 Torino (Italy) [email protected] Fedele F. G. 2014. — Camels, donkeys and caravan trade: an emerging context from Bara¯qish, ancient Yathill (Wa-di al-Jawf, Yemen). Anthropozoologica 49 (2): 177-194. http:// dx.doi.org/10.5252/az2014n2a02. ABSTRACT Work at Barāqish/Yathill in 2005-06 has produced sequences encompassing the Sabaean (13th-6th centuries BC) and Minaean/Arab (c. 550 BC-AD 1) occupa- tions. Abundant animal remains were retrieved and contexts of use and discard were obtained. Camels and donkeys are studied together as pack animals, the camel being the domestic dromedary. Their zooarchaeological and contextual study at Yathill is justified from this city’s location on the famous frankincense caravan route of the 1st millennium BC. An extramural stratigraphic sequence documenting the relationships between the city and the adjoining plain from c. 820 BC to the Islamic era was investigated to the northwest of the Minaean KEY WORDS wall. Domestic camels were present by 800 BC, the earliest well-documented Dromedary (Camelus occurrence in Yemen; wild dromedary herds were still in the area during the dromedarius), Camelus sp. wild, 7th century and perhaps later. The study of the archaeological context links donkey (Equus asinus), these Sabaean-age camels to campsites possibly formed by non-residents. This caravan trade, archaeological indicators pattern greatly developed during the Minaean period, with trade-jar handling of ‘caravan’ activity, posts outside the walled city and frequent stationing of camels and donkeys on ‘frankincense route’ in the upper talus. -

Harvard Polo Asia by Abigail Trafford
Horsing Around IN THE HIGHWAYS AND BYWAYS OF POLO IN ASIA We all meet up during the six-hour stopover in the Beijing Airport. The invitation comes from the Genghis Khan Polo Club to play in Mongolia and then to head back to China for a university tournament at the Metropolitan Polo Club in Tianjin. Say, what? Yes, polo! Both countries are resurrecting the ancient sport—a tale of two cultures—and the Harvard players are to be emissaries to help generate a new ballgame in Asia. In a cavernous airport restaurant, I survey the Harvard Polo Team: Jane is captain of the women’s team; Shawn, captain of the men’s team; George, the quiet one, is a physicist; Danielle, a senior is a German major; Sarah, a biology major; Aemilia writes for the Harvard Crimson. Marina, a mathematician, will join us later. Neil and Johann are incoming freshmen; Merrall, still in high school, is a protégé of the actor Tommy Lee Jones—the godfather of Harvard polo. And where are the grownups? Moon Lai, a friend of Neil’s parents, is the photographer from Minnesota. Crocker Snow, Harvard alum and head of the Edward R. Murrow Center at Tufts, is tour director and coach. I am along as cheer leader and chronicler. We stagger onto the late-night plane to Ulan Bator (UB), the capital of Mongolia, pile into a van and drive into the darkness—always in the constant traffic of trucks. Our first camp of log cabins is near an official site of Naadam—Mongolia’s traditional summer festival of horse racing, wrestling and archery. -

Evaluating and Comparing Descendants of Stallions from the Dark Ronald Line in Czech Warmblood Breeding According to Basic Body Measurements
11 1RYHPEHU 2020, Brno, Czech Republic Evaluating and comparing descendants of stallions from the Dark Ronald line in Czech Warmblood breeding according to basic body measurements Zuzana Kubikova, Iva Jiskrova, Barbora Kubistova Department of Animal Breeding Mendel University in Brno Zemedelska 1, 613 00 Brno CZECH REPUBLIC [email protected] Abstract: The aim of this study was to evaluate the influence of the Dark Ronald stallion line in the breeding of the Czech Warmblood. To evaluate the importance of the line, the offspring of stallions authorized to act as stud horses in the breeding of the Czech Warmblood were used. The values of the basic body measurements of their offspring (SHW, THW, ChC and CBC) were used to evaluate the quality of the stallions. A total of 6 breeding stallions were compared: two representatives of the Holsteiner horse (2765 CASSILIUS, 2666 PORTER), one Russian Holsteiner (2782 BALLAST), one Slovak Warmblood (794 CORSÁR), one Dutch Warmblood (2745 OSCAR) and one representative of the Zweibrücker breed (662 CARBIDO). The group for comparison consisted of 284 offspring of the Czech Warmblood breed from these sires. Evaluation by the GLM model detected statistically significant differences between the basic body measurements of the individual groups of offspring. The influence of the dams on the basic body measurements of offspring was taken into account by a graphical comparison of the massiveness and boniness indices. It was found that the stallion 2782 BALLAST significantly affects the massiveness of his offspring, but the dam has a greater influence on boniness than the sire. Furthermore, it was found that mares are statistically significantly more massive than stallions, but that they have a smaller cannon bone circumference. -

List of Horse Breeds 1 List of Horse Breeds
List of horse breeds 1 List of horse breeds This page is a list of horse and pony breeds, and also includes terms used to describe types of horse that are not breeds but are commonly mistaken for breeds. While there is no scientifically accepted definition of the term "breed,"[1] a breed is defined generally as having distinct true-breeding characteristics over a number of generations; its members may be called "purebred". In most cases, bloodlines of horse breeds are recorded with a breed registry. However, in horses, the concept is somewhat flexible, as open stud books are created for developing horse breeds that are not yet fully true-breeding. Registries also are considered the authority as to whether a given breed is listed as Light or saddle horse breeds a "horse" or a "pony". There are also a number of "color breed", sport horse, and gaited horse registries for horses with various phenotypes or other traits, which admit any animal fitting a given set of physical characteristics, even if there is little or no evidence of the trait being a true-breeding characteristic. Other recording entities or specialty organizations may recognize horses from multiple breeds, thus, for the purposes of this article, such animals are classified as a "type" rather than a "breed". The breeds and types listed here are those that already have a Wikipedia article. For a more extensive list, see the List of all horse breeds in DAD-IS. Heavy or draft horse breeds For additional information, see horse breed, horse breeding and the individual articles listed below. -

Genomics and the Evolutionary History of Equids Pablo Librado, Ludovic Orlando
Genomics and the Evolutionary History of Equids Pablo Librado, Ludovic Orlando To cite this version: Pablo Librado, Ludovic Orlando. Genomics and the Evolutionary History of Equids. Annual Review of Animal Biosciences, Annual Reviews, 2021, 9 (1), 10.1146/annurev-animal-061220-023118. hal- 03030307 HAL Id: hal-03030307 https://hal.archives-ouvertes.fr/hal-03030307 Submitted on 30 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annu. Rev. Anim. Biosci. 2021. 9:X–X https://doi.org/10.1146/annurev-animal-061220-023118 Copyright © 2021 by Annual Reviews. All rights reserved Librado Orlando www.annualreviews.org Equid Genomics and Evolution Genomics and the Evolutionary History of Equids Pablo Librado and Ludovic Orlando Laboratoire d’Anthropobiologie Moléculaire et d’Imagerie de Synthèse, CNRS UMR 5288, Université Paul Sabatier, Toulouse 31000, France; email: [email protected] Keywords equid, horse, evolution, donkey, ancient DNA, population genomics Abstract The equid family contains only one single extant genus, Equus, including seven living species grouped into horses on the one hand and zebras and asses on the other. In contrast, the equine fossil record shows that an extraordinarily richer diversity existed in the past and provides multiple examples of a highly dynamic evolution punctuated by several waves of explosive radiations and extinctions, cross-continental migrations, and local adaptations. -
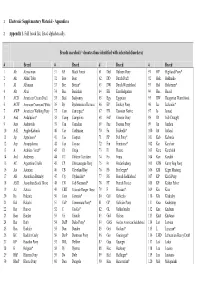
Electronic Supplementary Material - Appendices
1 Electronic Supplementary Material - Appendices 2 Appendix 1. Full breed list, listed alphabetically. Breeds searched (* denotes those identified with inherited disorders) # Breed # Breed # Breed # Breed 1 Ab Abyssinian 31 BF Black Forest 61 Dul Dülmen Pony 91 HP Highland Pony* 2 Ak Akhal Teke 32 Boe Boer 62 DD Dutch Draft 92 Hok Hokkaido 3 Al Albanian 33 Bre Breton* 63 DW Dutch Warmblood 93 Hol Holsteiner* 4 Alt Altai 34 Buc Buckskin 64 EB East Bulgarian 94 Huc Hucul 5 ACD American Cream Draft 35 Bud Budyonny 65 Egy Egyptian 95 HW Hungarian Warmblood 6 ACW American Creme and White 36 By Byelorussian Harness 66 EP Eriskay Pony 96 Ice Icelandic* 7 AWP American Walking Pony 37 Cam Camargue* 67 EN Estonian Native 97 Io Iomud 8 And Andalusian* 38 Camp Campolina 68 ExP Exmoor Pony 98 ID Irish Draught 9 Anv Andravida 39 Can Canadian 69 Fae Faeroes Pony 99 Jin Jinzhou 10 A-K Anglo-Kabarda 40 Car Carthusian 70 Fa Falabella* 100 Jut Jutland 11 Ap Appaloosa* 41 Cas Caspian 71 FP Fell Pony* 101 Kab Kabarda 12 Arp Araappaloosa 42 Cay Cayuse 72 Fin Finnhorse* 102 Kar Karabair 13 A Arabian / Arab* 43 Ch Cheju 73 Fl Fleuve 103 Kara Karabakh 14 Ard Ardennes 44 CC Chilean Corralero 74 Fo Fouta 104 Kaz Kazakh 15 AC Argentine Criollo 45 CP Chincoteague Pony 75 Fr Frederiksborg 105 KPB Kerry Bog Pony 16 Ast Asturian 46 CB Cleveland Bay 76 Fb Freiberger* 106 KM Kiger Mustang 17 AB Australian Brumby 47 Cly Clydesdale* 77 FS French Saddlebred 107 KP Kirdi Pony 18 ASH Australian Stock Horse 48 CN Cob Normand* 78 FT French Trotter 108 KF Kisber Felver 19 Az Azteca -

Correlations of Unfavorable Movement Characteristics in Warmblood Foals and Mares with Routinely Assessed Conformation and Performance Traits
Animal (2013), 7:1, pp 11–21 & The Animal Consortium 2012 animal doi:10.1017/S1751731112001322 Correlations of unfavorable movement characteristics in warmblood foals and mares with routinely assessed conformation and performance traits - A.-C. Becker1, K. F. Stock1,2 and O. Distl1 1Institute for Animal Breeding and Genetics, University of Veterinary Medicine Hannover, Buenteweg 17p, 30559 Hanover, Germany; 2Vereinigte Informationssysteme Tierhaltung w.V., Heideweg 1, 27283 Verden, Germany (Received 22 November 2011; Accepted 19 March 2012; First published online 6 July 2012) New movement traits reflecting unfavorable movement characteristics were defined on the basis of detailed movement evaluations (DME) of warmblood foals and mares performed in connection with regular breeding events of the Oldenburg horse breeding societies in 2009 and 2010. DME information was available for 3374 foals and 2844 mares and used for correlation analyses with conformation information on 1987 mares from studbook inspections (SBI) in 2009 and performance information on 2758 mares from mare performance tests (MPT) in 2000 to 2008. Analyses of variance revealed few significant differences between scores for SBI and MPT traits in mares without and with indications of imbalance (IMB) in general or specific findings like irregular tail tone or posture (TTP). SBI scores for general impression and development were significantly lower and MPT scores for trot under rider tended to be higher in IMB-positive mares. Genetic parameters were estimated in linear animal models with residual maximum likelihood. Additive genetic correlations and Pearson correlation coefficients between univariately predicted breeding values indicated unfavorable genetic correlations of IMB and TTP with dressage-related conformation and performance traits. -

ECOCYCLES Open Access Scientific Journal ISSN 2416-2140 of the European Ecocycles Society
ECOCYCLES Open access scientific journal ISSN 2416-2140 of the European Ecocycles Society Ecocycles, Vol. 5, No. 1, pp. 67-97 (2019) DOI: 10.19040/ecocycles.v5i1.156 ORIGINAL ARTICLE hose policy will win the b Equestrian tourism and horse breeding in Hungary and Slovenia – environmental sustainability and conservation of cultural heritage: a strategic approach Sándor Némethy1,2 and Ádám Bartos3 1 University of Pécs, Institute of Regional Development, Hungary; 2 University of Gothenburg, Dept. of Conservation, Sweden; 3Georgikon Faculty, University of Pannonia, Keszthely, Hungary Abstract – Historically, horse-breeding and riding has been an integral part of Hungarian and Slovenian culture for over a thousand years. In a broader sense, the equestrian sector includes all related activities, without which the efficient operation of the sector is unthinkable. Examples include infrastructure development, fodder production, veterinary services, the institutional system operating in the sector (public and non-governmental organizations, etc.). In the narrower sense, the equestrian industry is the sum of all areas where the horse is the main driver of its operation. This includes all areas of horse-related activities, such as all-inclusive education, use of horses in organic agriculture, horse breeding, equestrian tourism, horse racing, traditional historic horse-events, equestrian therapy, recreational riding and horseback riding are key elements. A feasibility study was carried out in the Hungarian – Slovenian border region to explore the possibilities for joint cross-boundary development of horse- based tourism. Hungary's and Slovenia’s contemporary natural qualities provide excellent opportunities for equestrian tourism. The starting point for formulating cross-boundary equestrian programme is that the mutually reinforcing, complex and holistic development of each sub-area can only produce results. -

Paleogenomics of Animal Domestication
Paleogenomics of Animal Domestication Evan K. Irving-Pease, Hannah Ryan, Alexandra Jamieson, Evangelos A. Dimopoulos, Greger Larson, and Laurent A. F. Frantz Abstract Starting with dogs, over 15,000 years ago, the domestication of animals has been central in the development of modern societies. Because of its importance for a range of disciplines – including archaeology, biology and the humanities – domestication has been studied extensively. This chapter reviews how the field of paleogenomics has revolutionised, and will continue to revolutionise, our under- standing of animal domestication. We discuss how the recovery of ancient DNA from archaeological remains is allowing researchers to overcome inherent shortcom- ings arising from the analysis of modern DNA alone. In particular, we show how DNA, extracted from ancient substrates, has proven to be a crucial source of information to reconstruct the geographic and temporal origin of domestic species. We also discuss how ancient DNA is being used by geneticists and archaeologists to directly observe evolutionary changes linked to artificial and natural selection to generate a richer understanding of this fascinating process. Keywords Ancient DNA · Archaeology · Domestication · Entomology · Evolution · Genomics · Zoology E. K. Irving-Pease (*) · H. Ryan · A. Jamieson · E. A. Dimopoulos · G. Larson The Palaeogenomics and Bio-Archaeology Research Network, Research Laboratory for Archaeology and History of Art, University of Oxford, Oxford, UK e-mail: [email protected] L. A. F. Frantz (*) The Palaeogenomics and Bio-Archaeology Research Network, Research Laboratory for Archaeology and History of Art, University of Oxford, Oxford, UK School of Biological and Chemical Sciences, Queen Mary University of London, London, UK e-mail: [email protected] Charlotte Lindqvist and Om P. -

Genetics and Animal Domestication: New Windows on an Elusive Process K
Journal of Zoology. Print ISSN 0952-8369 Genetics and animal domestication: new windows on an elusive process K. Dobney1 & G. Larson2 1 Department of Archaeology, University of Durham, Durham, UK 2 Department of Zoology, Henry Wellcome Ancient Biomolecules Centre, University of Oxford, UK Keywords Abstract domestication; genetics; phylogeography; molecular clocks; paedomorphosis. Domesticated animals are universally familiar. How, when, where and why they became domesticated is less well understood. The genetic revolution of the past few Correspondence decades has facilitated novel insights into a field that previously was principally the Greger Larson, Department of Zoology, domain of archaeozoologists. Although some of the conclusions drawn from Henry Wellcome Ancient Biomolecules genetic data have proved to be contentious, many studies have significantly altered Centre, University of Oxford, South Parks or refined our understanding of past human animal relationships. This review Road OX1 3PS, UK seeks not only to discuss the wider concerns and ramifications of genetic Email: [email protected] approaches to the study of animal domestication but also to provide a broader theoretical framework for understanding the process itself. More specifically, we Received 6 July 2005; accepted 8 September discuss issues related to the terminology associated with domestication, the 2005 possibility of domestication genes, and the promise and problems of genetics to answer the fundamental questions associated with domestication. doi:10.1111/j.1469-7998.2006.00042.x Introduction Defining domestication Over the past 10 000 years, human history has been wholly Terminology typically used in domestication studies, including transformed by the domestication of plants and animals. the word ‘domestication’ itself, is often confusing and poorly Although the term ‘domestic animal’ has universal meaning, defined.