December 14, 1997
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Media Guide.Qxd
2006 OHL PRIORITY SELECTION MEDIA GUIDE OHL PRIORITY SELECTION • MAY 6, 2006 On May 5 2001, the Ontario Hockey League conducted the annual Priority Selection process by way of the Internet for the first time in league history. The league web site received record traffic for the single day event, topping 140,000 visitor sessions and 1.8 million page views. The 2006 OHL Priority Selection will once again be conducted online on Saturday May 6, 2006 beginning at 9:00 a.m. at www.ontariohockeyleague.com. This media guide has been prepared as a resource to all media covering the 2006 OHL Priority Selection. Additional media resources, including player head and shoulders photos and draft day informa- tion will be posted on the league’s media information web site - www.ontariohockeyleague.com/media . Contents Team Contact Information 3 Player Eligibility 4 Order of Selection 5 OHL Central Scouting 6 Jack Ferguson Award 6 Selected Player Profiles 7 Eligible Player List 12 Eligible Player List - Goaltenders 21 First Round Draft Picks 22 2005 Priority Selection Results by Team 25 2004 Priority Selection Results by Team 27 2003 Priority Selection Results by Team 29 2002 Priority Selection Results by Team 31 2001 Priority Selection Results by Team 33 2000 Priority Selection Results by Team 35 1999 Priority Selection Results by Team 37 1998 Priority Selection Results by Team 40 1997 Priority Selection Results by Team 42 2 TEAM CONTACT INFO Barrie Colts Ottawa 67’s 555 Bayview Drive, Barrie, ON L4N 8Y2 1015 Bank Street Gate #4 , Ottawa, ON K1S 3W7 Phone: 705/722-6587 Fax: 705/721-9709 Phone: 613/232-6767 Fax: 613/232-5582 [email protected] / www.barriecolts.com [email protected] / www.ottawa67s.com GM - Mike McCann; PR - Jason Ford GM - Brian Kilrea; PR - Bryan Cappell Belleville Bulls Owen Sound Attack 265 Cannifton Road, Belleville, ON K8N 4V8 1900 3rd Ave. -

Press Clips November 25, 2015
Buffalo Sabres Daily Press Clips November 25, 2015 Predators-Sabres Preview AP November 25, 2015 The Buffalo Sabres have found goals hard to come by during a season-high, five-game slide. The Nashville Predators won't have any sympathy as they endure the longest scoring drought in club history after being shut out three straight times. These teams with floundering attacks meet Wednesday night, with the visiting Predators seeking a fifth straight win in the series. Buffalo (8-11-2) fell 2-1 at home to St. Louis on Monday to drop to 0-3-2 in its last five. The Sabres took the lead into the third period on former Predators center David Legwand's first goal only to falter over the final 20 minutes. "I thought it was a hard-fought game," coach Dan Bylsma said. "There was a lot of good in a lot of areas from our team. You'd like to find some goals in there." Bylsma's club isn't the only one searching for goals with Nashville (11-6-3) enduring a drought of 213 minutes, 47 seconds after Monday's 3-0 road loss to the New York Rangers. That's the longest in the NHL since the Coyotes were blanked for 245:33 in 2012-13. The Predators are on the verge of even more dubious distinctions. The last time a team was shut out four straight times happened to the 1967-68 Oakland Seals, who tied one of those games. The last club to lose by shutout four straight times was the Montreal Maroons, whose streak spanned the end of 1929-30 and beginning of 1930-31. -

June 18, 2000
lomeTbwn COMMUNICATIONS NETWORK Ulestlani) (Dbserwr Your hometown newspaper serving Westland for 36 years aW ^aw Sunday, June 18, 2000 hometownnewspapers.net 75C Volume 36 Number 5 Wastlang, MteNoan OeOOo HomeTown Communicator* Natwof«4t Glad you're my dad Victim feared for life • In emotional testimony Thursday, a woman described a brutal assault in Westland. Charges include attempted murder. BY DAHHELL CLEM 8TAWWWITO dcIea>Ao«JtoiB««oiUBUiet Raped, beaten and crawling on soggy ground in a dark, wooded area of West- land, a 48-year-old woman feared she was going to be killed when her attack er got into his pickup truck and started aurr Pacma n ft* MA*UY the engine. THE WEEK "To myself I said, 'He's going to run Thanksl Above, Valerie over me with his truck/ "the victim Poma, 2% of Westland testified Thursday. "I thought he was holds the picture frame she going to kill me because of the blows f^Wmmmg^Lwdf made for her dad, David, and the strikes and the way he was' for Father's Day at the beating me. I thought, This is it.'" Westland library this past Instead, she said, her attacker drove off after he forced her to perform oral MONDAY week. With Valerie in the sex, raped her inside his truck, and photo when she was a baby beat and kicked her so brutally that, is older sister Melissa. At she still winced in pain Thursday from City Hall: The Westland right, Darcy Vines, 5, of broken vertebrae and ribs she suffered City Council will meet 7 Westland works on the pic May 29. -
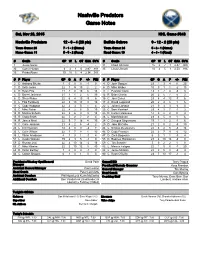
Nashville Predators Game Notes
Nashville Predators Game Notes Sat, Nov 28, 2015 NHL Game #348 Nashville Predators 12 - 6 - 4 (28 pts) Buffalo Sabres 9 - 12 - 2 (20 pts) Team Game: 23 7 - 1 - 2 (Home) Team Game: 24 5 - 8 - 1 (Home) Home Game: 11 5 - 5 - 2 (Road) Road Game: 10 4 - 4 - 1 (Road) # Goalie GP W L OT GAA SV% # Goalie GP W L OT GAA SV% 1 Juuse Saros - - - - - - 31 Chad Johnson 15 5 7 1 2.47 .909 30 Carter Hutton 3 2 1 0 2.97 .911 35 Linus Ullmark 10 4 5 1 2.50 .916 35 Pekka Rinne 19 10 5 4 2.34 .911 # P Player GP G A P +/- PIM # P Player GP G A P +/- PIM 2 D Anthony Bitetto 1 0 0 0 -1 0 4 D Josh Gorges 23 1 3 4 0 25 3 D Seth Jones 22 1 9 10 2 2 6 D Mike Weber 10 0 1 1 -2 13 4 D Ryan Ellis 21 2 8 10 5 14 9 L Evander Kane 13 2 2 4 -5 8 5 D Barret Jackman 21 1 1 2 5 39 12 R Brian Gionta 20 2 5 7 -5 2 6 D Shea Weber 22 6 4 10 -5 6 15 C Jack Eichel 23 8 4 12 -7 6 9 L Filip Forsberg 22 3 10 13 3 16 17 C David Legwand 20 2 4 6 1 6 11 C Cody Hodgson 22 2 3 5 1 4 22 L Johan Larsson 21 0 3 3 -3 0 12 C Mike Fisher 22 4 2 6 0 19 23 C Sam Reinhart 23 4 3 7 -1 2 14 D Mattias Ekholm 22 3 6 9 3 14 25 D Carlo Colaiacovo 14 0 2 2 0 4 15 R Craig Smith 22 5 2 7 2 4 26 L Matt Moulson 23 4 5 9 1 6 18 R James Neal 22 9 7 16 4 33 28 C Zemgus Girgensons 19 1 1 2 -1 6 19 C Calle Jarnkrok 21 4 2 6 -4 2 29 D Jake McCabe 21 2 0 2 -5 10 24 L Eric Nystrom 14 3 0 3 -3 7 44 L Nicolas Deslauriers 22 3 2 5 -4 14 33 L Colin Wilson 22 1 7 8 1 10 46 D Cody Franson 23 2 7 9 -3 12 38 L Viktor Arvidsson 4 1 0 1 -1 4 47 D Zach Bogosian 6 0 1 1 -3 4 51 L Austin Watson 19 2 3 5 -1 9 55 D Rasmus Ristolainen -

2007 SC Playoff Summaries
CHICAGO BLACKHAWKS STANLEY CUP CHAMPIONS 2 0 1 0 Dave Bolland, Nick Boynton, Troy Brouwer, Adam Burish, Dustin Byfuglien, Brian Campbell, Ben Eager, Colin Fraser, Jordan Henry, Niklas Hjalmarsson, Marian Hossa, Cristobal Huet, Patrick Kane, Duncan Keith, Tomas Kopecky, Andrew Ladd, John Madden, Antti Niemi, Brent Seabrook, Patrick Sharp, Brent Sopel, Jonathan Toews CAPTAIN, Kris Versteeg Stan Bowman GENERAL MANAGER, Joel Quenneville HEAD COACH © Steve Lansky 2010 bigmouthsports.com NHL and the word mark and image of the Stanley Cup are registered trademarks and the NHL Shield and NHL Conference logos are trademarks of the National Hockey League. All NHL logos and marks and NHL team logos and marks as well as all other proprietary materials depicted herein are the property of the NHL and the respective NHL teams and may not be reproduced without the prior written consent of NHL Enterprises, L.P. Copyright © 2010 National Hockey League. All Rights Reserved. 2010 EASTERN CONFERENCE QUARTER—FINAL 1 WASHINGTON CAPITALS 121 v. 8 MONTRÉAL CANADIENS 88 GM GEORGE McPHEE, HC BRUCE BOUDREAU v. GM PIERRE GAUTHIER, HC JACQUES MARTIN CANADIENS WIN SERIES IN 7 Thursday, April 15 1900 h et on TSN, Versus Saturday, April 17 1900 h et on TSN, Versus MONTREAL 3 @ WASHINGTON 2 OVERTIME MONTREAL 5 @ WASHINGTON 6 OVERTIME FIRST PERIOD FIRST PERIOD 1. MONTREAL, Mike Cammalleri 1 (Andrei Markov, Andrei Kostitsyn) 12:36 PPG 1. MONTREAL, Brian Gionta 1 (Scott Gomez) 1:00 2. WASHINGTON, Joe Corvo 1 (Eric Belanger, Jason Chimera) 15:33 2. MONTREAL, Andrei Kostitsyn 1 (unassisted) 7:58 3. WASHINGTON, Eric Fehr 1 (Tomas Fleischmann) 10:21 Penalties ― Bergeron M 0:52, Semin W 6:01, Backstrom W 12:11, Pouliot M 12:53 Penalties ― None SECOND PERIOD NO SCORING SECOND PERIOD 4. -

Lightning Down Philadelphia Flyers
Sports FRIDAY, NOVEMBER 29, 2013 Lightning down Philadelphia Flyers TAMPA: Victor Hedman had two goals and an beat Los Angeles to extend the home-team Iginla scored for Boston and Tuukka Rask Markov had three assists and Montreal’s NHL- assist to help the Tampa Bay Lightning beat dominance in this California rivalry. Thornton stopped 22 shots. Detroit scored three goals best road power play scored twice in four the Philadelphia Flyers 4-2 on Wednesday and Joe Pavelski scored in regulation for the in less than 4 minutes in the second period. chances. Matt Moulson scored for Buffalo as night, spoiling Vincent Lecavalier’s homecom- Sharks, who have won nine straight at home the Sabres lost their fifth consecutive game in ing. Lecavalier, who played 14 years for the against the Kings including the postseason. In RANGERS 5, PANTHERS 2 regulation. Ryan Miller made 28 saves. Lightning after being selected first overall in all, the home team has won the last 14 Rick Nash scored his second goal of the the 1998 draft, faced his old team for the first matchups, including all seven in last spring’s season and Henrik Lundqvist rebounded from HURRICANES 4, DEVILS 3 time and scored a power-play goal late in the playoff series won by Los Angeles. Jeff Carter a poor game with 31 saves to lift New York Patrick Dwyer scored short-handed to cap third period. The former Tampa Bay captain and Drew Doughty scored for the Kings, who over Florida. Mats Zuccarello, Brad Richards a three-goal burst in the second period, and received a standing ovation after a video trib- tied a franchise record by earning a point in and Derick Brassard also scored for the Carolina snapped its six-game road losing ute was played on the scoreboard midway their 11th straight game. -

Life and Times" Video Recordings
http://oac.cdlib.org/findaid/ark:/13030/c8qr4zn7 No online items KCET-TV Collection of "Life and Times" video recordings Taz Morgan William H. Hannon Library Loyola Marymount University One LMU Drive, MS 8200 Los Angeles, CA 90045-8200 Phone: (310) 338-5710 Fax: (310) 338-5895 Email: [email protected] URL: http://library.lmu.edu/collections/archivesandspecialcollections/ ©2013 Loyola Marymount University. All rights reserved. KCET-TV Collection of "Life and CSLA-37 1 Times" video recordings KCET-TV Collection of "Life and Times" video recordings Collection number: CSLA-37 William H. Hannon Library Loyola Marymount University Los Angeles, California Processed by: Taz Morgan Date Completed: October 2013 Encoded by: Taz Morgan 2013 Loyola Marymount University. All rights reserved. Descriptive Summary Title: KCET-TV Collection of "Life and Times" video recordings Dates: 1991-2007 Collection number: CSLA-37 Creator: KCET (Television station : Los Angeles, Calif.) Collection Size: 3,472 videotapes (332 boxes) Repository: Loyola Marymount University. Library. Department of Archives and Special Collections. Los Angeles, California 90045-2659 Languages: Languages represented in the collection: English Access Collection is open to research under the terms of use of the Department of Archives and Special Collections, Loyola Marymount University. Duplication of program tapes for research use is required in accordance with departmental policy regarding the formats of the videotapes of this collection: "Certain media formats may need specialized third party vendor services. If the department does not own a researcher access copy (DVD copy), the cost of reproduction, to be paid fully by patron, will include 1) any necessary preservation efforts upon the original, 2) a master file to be retained by Archives and Special Collections, 3) a researcher viewing copy to be retained by Archives and Special Collections, and 4) the patron copy. -

OHL Priority Selection Preview and Media Guide:OHL News.Qxd
OHL PRIORITY SELECTION OHL Priority Selection Process In 2001, the Ontario Hockey League Selected Players in the OHL with non-playoff teams selecting ahead Scouting Bureau with evaluations from conducted the annual Priority Selec- OHL Member Teams are permitted to of playoff teams. their team scouting staffs to make their tion process by way of the Internet for register a maximum of four 16 year old player selections. the first time in league history. players selected in the OHL Priority Teams are permitted to trade draft Selection. Those 16 year old players choices, other than their first round se- The OHL Central Scouting Bureau The new process allowed for eligible that are allowed to be signed are the lection, during the trading period from has been evaluating players since the players and their families, as well as fans first two 16 year old players selected Monday April 28 to Friday May 2, 1975-76 season. across the league to follow the process and a maximum addition of two 16 2008 at 3:00 p.m. in real time online. year old wild carded players in any OHL Central Scouting Staff round of the OHL Priority Selection. OHL Central Scouting Chief Scout - Robert Kitamura The 2008 OHL Priority Selection will The Central Scouting Bureau of the GTA - Tim Cherry once again be conducted online on All other 16-year-old players selected Ontario Hockey League is an informa- Central Ontario - Kyle Branch Saturday May 3, 2008 beginning at are eligible to be called up as an “affili- tion service and support organization Kingston and Area - John Finlay 9:00 a.m. -

In Oklahoma P 'Pastor, Victim of Crash Occurred on Highway in Church Planning Oklahoma City Memoricl.L Service Ilr Spite of Djfricl1jtips Rc' ..,Ulting Rq
!' I i' , il 11 1 r ,,° I II RACD , 1 IServices Held In Oklahoma p 'Pastor, Victim of Crash Occurred On Highway in Church planning Oklahoma City Memoricl.l Service Ilr spite of djfricl1Jtips rC' ..,ulting Rq.:. Walter A. Brackcnsick, ~:? ~~~aUi1~r~{d 1r~f\Sdj~;~~~~~1 \~~~l~~~t: r~S~~y~~'~~~~('~~~i~~ertt~n~~~r~;~ what it hopes, is a fairly accurate' Me'n1Qrial Park femetery at Oldd- ~~hci~~n~i?I~~h~('av~I~?tb)~e ~~c~~C~~~ ;\~)~Hlp;~I\~~~r~~' ha~ldhl~ ~~~~~~:i .<'lck, his mother-in-Jaw and sislcr- srrvi('(' for hi) here aft(', Mrs.. in-law. ' Bnwlu'ns.ick is hie to relurn· to The Braekcnsicks w('re in Okla- Wayne. , hom a on a two-w('pk vaca!tlOn A trAgIC .lCClfi!"'nl on Ii hlg~\\d.7 They fIrst \\('nl 10 Chle:J.~o and In ()klahoma C ty On Weclne$;dBY then to Quincy. Illf, Ihe IMstor s ('\..(>nmg. Aug 4~ClalmCd the, Illr hirthplilce. From QUincy.. they of fhC' Wayne Jnister apd als!! drove to Oklahomal CIty 10 VI~'L fatally m!Ured rs Brackenc:ack '\ l-e~~~ivr~COff'~;;i'n~r~~k~~~~~~Sday, ~n~;I~;s ~.~~ll~I~ G~!~e~tc;\s7~i~ I 1 I Ailg'. 4, Rev, and Mrs Bl<~cken· of Mr" Bracken lck ' sick. daught('r, Dorotl"\y, and son nev Bratckrn ci{ and Mrs Gr.t- ' James, accomp'Ini('d hy Mr~ aml nnw \" .. cn klllr I Instantly, 1\1r"" ,', ouhty Mrs. Ca, rl Granow, Mrs Emma i<uf'nk( I d!f'd 11 hours later m dn t .... -
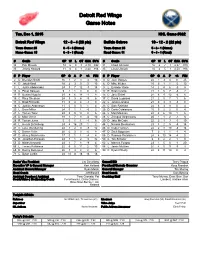
Detroit Red Wings Game Notes
Detroit Red Wings Game Notes Tue, Dec 1, 2015 NHL Game #362 Detroit Red Wings 12 - 8 - 4 (28 pts) Buffalo Sabres 10 - 12 - 2 (22 pts) Team Game: 25 6 - 5 - 3 (Home) Team Game: 25 5 - 8 - 1 (Home) Home Game: 15 6 - 3 - 1 (Road) Road Game: 11 5 - 4 - 1 (Road) # Goalie GP W L OT GAA SV% # Goalie GP W L OT GAA SV% 34 Petr Mrazek 14 6 4 3 2.19 .932 31 Chad Johnson 16 6 7 1 2.37 .913 35 Jimmy Howard 11 6 4 1 2.40 .914 35 Linus Ullmark 10 4 5 1 2.50 .916 # P Player GP G A P +/- PIM # P Player GP G A P +/- PIM 2 D Brendan Smith 16 1 2 3 3 10 4 D Josh Gorges 24 1 3 4 0 25 4 D Jakub Kindl 18 2 3 5 3 10 6 D Mike Weber 10 0 1 1 -2 13 8 L Justin Abdelkader 24 5 7 12 -3 18 9 L Evander Kane 14 2 4 6 -3 8 13 C Pavel Datsyuk 9 2 1 3 0 0 12 R Brian Gionta 21 2 5 7 -5 2 14 R Gustav Nyquist 24 8 6 14 -1 8 15 C Jack Eichel 24 8 4 12 -6 6 15 C Riley Sheahan 24 3 3 6 -5 6 17 C David Legwand 21 2 5 7 1 6 17 C Brad Richards 11 0 4 4 -1 0 22 L Johan Larsson 21 0 3 3 -3 0 18 C Joakim Andersson 11 0 0 0 1 4 23 C Sam Reinhart 24 6 3 9 0 4 20 L Drew Miller 24 0 1 1 -5 2 25 D Carlo Colaiacovo 15 0 2 2 0 4 21 L Tomas Tatar 24 8 9 17 0 6 26 L Matt Moulson 24 4 6 10 1 6 25 D Mike Green 18 1 7 8 -6 10 28 C Zemgus Girgensons 20 1 1 2 -1 8 26 R Tomas Jurco 5 0 1 1 -1 6 29 D Jake McCabe 22 2 1 3 -3 10 40 L Henrik Zetterberg 24 4 16 20 1 2 44 L Nicolas Deslauriers 23 3 2 5 -4 14 41 C Luke Glendening 24 1 2 3 -9 12 46 D Cody Franson 24 2 8 10 -3 14 43 C Darren Helm 20 0 3 3 -6 8 47 D Zach Bogosian 7 0 1 1 -1 4 47 D Alexey Marchenko 17 0 1 1 -4 4 55 D Rasmus Ristolainen 24 4 -

Creative Industries in South Korea: the Korean Wave
CREATIVE INDUSTRIES IN SOUTH KOREA: THE KOREAN WAVE Author: Nicoleta Stefanÿ Valean Tutor: Francesc Xavier Molina Morales DEGREE IN BUSINESS ADMINISTRATION AE1049 - FINAL PROJECT WORK ACADEMIC YEAR: 2016/2017 CREATIVE INDUSTRIES IN SOUTH KOREA: THE KOREAN WAVE TABLE OF CONTENTS INTRODUCTION 3 1. CREATIVE INDUSTRY 5 1.1. Definition. 5 1.2. Origin. 5 2. SOUTH KOREA 6 2.1. The history of Korea. 6 2.2. Hallyu: The Korean Wave 9 2.3. Aspects related to Hallyu 13 2.3.1. Industry Policy 14 2.3.2. Hallyu’s Kdramas approach 15 2.3.3. Hallyu and National Prestige 16 2.3.4. Market Segmentation 18 3. KOREAN POPULAR CULTURE 20 3.1. Korean television and Kpop 20 3.2. The Big Three: SM, YG and JYP 24 3.2.1. SM Entertainment 25 3.2.2. YG Entertainment 28 3.2.3 JYP Entertainment 29 3.2.4. Trainee system 31 4. CONCLUSION 33 5. REFERENCES 34 6. WEBGRAPHY 36 2 CREATIVE INDUSTRIES IN SOUTH KOREA: THE KOREAN WAVE INTRODUCTION We live in a globalized world, surrounded by the effects of globalization in our daily life. Nowadays we have access to information about so many different cultures, countries, economies, different organizations, and so on. Thanks to the Internet, we have access to a whole new world in just a click. This is the main characteristic of the actual global situation. Personally, I am always amazed of this fact, being able to “travel" with just a click, being able to communicate with someone on the other side of the world, being able to know exactly what is happening, for example, in Australia while being in Spain, and more. -

278 Oral Abstract Session, Thu, 1:30 PM-3:00 PM Results of The
ESOPHAGEAL AND GASTRIC CANCER 278 Oral Abstract Session, Thu, 1:30 PM-3:00 PM Results of the JAVELIN Gastric 100 phase 3 trial: avelumab maintenance following first-line (1L) chemotherapy (CTx) vs continuation of CTx for HER22 advanced gastric or gastroesophageal junction cancer (GC/GEJC). Markus H. Moehler, Mikhail Dvorkin, Mustafa Ozguroglu, Min-hee Ryu, Alina Simona Muntean, Sara Lonardi, Marina Nechaeva, Arinilda Silva Campos Bragagnoli, Hasan Senol Coskun, Antonio Cubillo Graci´an, Toshimi Takano, Rachel Wong, Howard Safran, Gina M. Vaccaro, Narikazu Boku, Ilaria Conti, Janet Hong, Huiling Xiong, Julien Taieb, Yung-Jue Bang; Johannes Gutenberg-University Clinic, Mainz, Germany; Omsk Regional Clinical Centre of Oncology, Omsk, Russian Federation; Cerrahpas¸a School of Medicine, Istanbul University-Cerrahpas¸a, Istanbul, Turkey; Department of Oncology, Asan Medical Center, Seoul, South Korea; "Prof. Dr. Ion Chiricut¸a"˘ Institute of Oncology, Cluj-Napoca, Romania; Medical Oncology Unit 1, Clinical and Experimental Oncology Department, Veneto Institute of Oncology IOV–IRCCS, Padua, Italy; Clinical Oncology Dispensary, Arkhangelsk, Russian Federation; Barretos Cancer Hospital, Barretos, Brazil; Akdeniz University, Antalya, Turkey; HM Universitario Sanchinarro, Madrid, Spain; Department of Medical Oncology, Toranomon Hospital, Tokyo, Japan; Eastern Health, Monash University, Melbourne, Australia; Brown University Oncology Research Group, Providence, RI; Oregon Health & Science University, Portland, OR; National Cancer Center Hospital, Tokyo, Japan; EMD Serono, Billerica, MA; Merck Serono, Beijing, China; Hopitalˆ Europ´een Georges-Pompidou, Sorbonne Paris Cite/Paris Descartes University, Paris, France; Seoul National University Hospital, Seoul, South Korea Background: We report the primary analysis of JAVELIN Gastric 100, which compared avelumab (anti–PD-L1) maintenance after 1L CTx vs continued CTx in patients (pts) with GC/GEJC.