For Peer Review Only Journal: Expert Opinion on Therapeutic Targets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluation of Perennial Ryegrass Straw As a Forage Source for Ruminants
AN ABSTRACT OF THE THESIS OF Michael J. Fisher for the degree of Master of Science in Animal Sciencepresented on July 28, 2003. Title: Evaluation of Perennial Ryegrass Strawas a Forage Source for Ruminants. Redacted for Privacy Abstract approved: David W. Bohnert We conducted two experiments evaluating perennialryegrass straw as a forage source for ruminants. Experiment 1 evaluated digestion and physiological variables in steers offered perennial ryegrass straw containing increasing levels of lolitrem B. Sixteen ruminally cannulated Angus X Hereford steers (231 ± 2 kg BW)were blocked by weight and assigned randomly to one of four treatments (TRT). Steerswere provided perennial ryegrass straw at 120% of the previous 5-daverage intake. Prior to straw feeding, soybean meal (SBM) was provided (0.1% BW; CP basis) tomeet the estimated requirement for degradable intake protein. Low (L) and high(H) lolitrem B straws (<100 and 1550 ppb, respectively) were used to formulate TRT diets: LOW (100% L); LOW MIX (67% L:33% H); HIGH MIX (33% L:67% H); HIGH(100% H). Intake and digestibility of DM and OM, and ruminal pH, total VFA, andNH3-N were not affected by increasing lolitrem B concentration (P> 0.13). Ruminal indigestible ADF (IADF) fill increased linearly (P= 0.01) and JADF passage rate (%/h) decreased linearly (P = 0.04) as lolitrem B level increased. Experiment2 evaluated performance and production of 72 Angus X Herefordcows (539 ± 5 kg BW) consuming perennial ryegrass straw containing increasing levels of lolitremB during the last third of gestation. Cowswere blocked by body condition score (BCS) and randomly assigned to one of three TRT. -

Interactions of Insecticidal Spider Peptide Neurotoxins with Insect Voltage- and Neurotransmitter-Gated Ion Channels
Interactions of insecticidal spider peptide neurotoxins with insect voltage- and neurotransmitter-gated ion channels (Molecular representation of - HXTX-Hv1c including key binding residues, adapted from Gunning et al, 2008) PhD Thesis Monique J. Windley UTS 2012 CERTIFICATE OF AUTHORSHIP/ORIGINALITY I certify that the work in this thesis has not previously been submitted for a degree nor has it been submitted as part of requirements for a degree except as fully acknowledged within the text. I also certify that the thesis has been written by me. Any help that I have received in my research work and the preparation of the thesis itself has been acknowledged. In addition, I certify that all information sources and literature used are indicated in the thesis. Monique J. Windley 2012 ii ACKNOWLEDGEMENTS There are many people who I would like to thank for contributions made towards the completion of this thesis. Firstly, I would like to thank my supervisor Prof. Graham Nicholson for his guidance and persistence throughout this project. I would like to acknowledge his invaluable advice, encouragement and his neverending determination to find a solution to any problem. He has been a valuable mentor and has contributed immensely to the success of this project. Next I would like to thank everyone at UTS who assisted in the advancement of this research. Firstly, I would like to acknowledge Phil Laurance for his assistance in the repair and modification of laboratory equipment. To all the laboratory and technical staff, particulary Harry Simpson and Stan Yiu for the restoration and sourcing of equipment - thankyou. I would like to thank Dr Mike Johnson for his continual assistance, advice and cheerful disposition. -

Holocene Environmental Changes Disclosed from Anoxic Fjord Sediments by Biomarkers and Their Radiocarbon Content
GEOLOGICA ULTRAIECTINA Mededelingen van de Faculteit Geowetenschappen Universiteit Utrecht No. 227 Holocene environmental changes disclosed from anoxic fjord sediments by biomarkers and their radiocarbon content Rienk H. Smittenberg Holocene environmental changes disclosed from anoxic fjord sediments by biomarkers and their radiocarbon content Holocene milieuveranderingen gereconstrueerd uit anoxische fjord sedimenten middels biomarkers en hun radio-aktief koolstof gehalte (met een samenvatting in het Nederlands) Proefschrift ter verkrijging van de graad van doctor aan de Universiteit Utrecht op gezag van de Rector Magnificus, Prof. Dr. W.H. Gispen, ingevolge het besluit van het College voor Promoties in het openbaar te verdedigen op maandag 15 september 2003 des middags te 4.15 uur door Rienk Hajo Smittenberg geboren op 23 maart 1973 te Eck en Wiel Promotor: Prof. Dr. J.W. de Leeuw Department of Geochemistry Utrecht University Utrecht, The Netherlands Copromotores: Dr. Ir. J.S. Sinninghe Damsté Department of Geochemistry Utrecht University Utrecht, The Netherlands Dr. S. Schouten Department of Marine Biogeochemistry and Toxicology Royal Netherlands Institute of Sea Research Texel, The Netherlands The research described in this thesis was carried out at the Department of Biogeochemistry and Toxicology of the Royal Netherlands Institute of Sea Research, P.O. Box 59, 1790 AB Den Burg, The Netherlands. The investigations were supported by the Research Council for Earth and Life Science (ALW) with the financial support from the Netherlands -
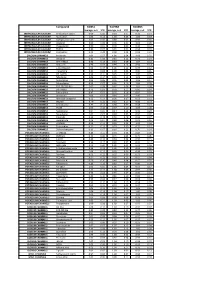
Biomol Average and SD Table S1.Xlsx
Compound GliNS1 G179NS G166NS average, n=5 STD average, n=3 STD average, n=3 STD INTRACELLULAR CALCIUM Antibiotic A-23187 0.00 0.00 0.10 0.07 0.15 0.14 INTRACELLULAR CALCIUM Ryanodine 1.04 0.14 1.03 0.03 1.03 0.03 INTRACELLULAR CALCIUM Cyclopiazonic acid 1.01 0.06 0.88 0.05 0.92 0.06 INTRACELLULAR CALCIUM Gingerol 1.00 0.06 0.91 0.01 1.01 0.06 INTRACELLULAR CALCIUM Thapsigargin 0.00 0.01 0.00 0.00 0.10 0.12 INTRACELLULAR CALCIUM TMB-8 0.89 0.07 0.91 0.05 0.94 0.03 INTRACELLULAR CALCIUM Dantrolene 0.91 0.08 0.98 0.05 0.94 0.01 CALCIUM CHANNELS Amiloride 1.01 0.07 1.01 0.04 1.03 0.05 CALCIUM CHANNELS Benzamil 0.83 0.08 0.83 0.12 0.96 0.04 CALCIUM CHANNELS BAY K-8644 0.93 0.13 0.93 0.09 1.07 0.14 CALCIUM CHANNELS Diltiazem 0.96 0.07 0.99 0.12 0.94 0.14 CALCIUM CHANNELS L-cis-Diltiazem 0.91 0.17 1.01 0.12 0.95 0.12 CALCIUM CHANNELS Flunarizine 0.85 0.08 1.00 0.06 0.85 0.05 CALCIUM CHANNELS FPL-64176 0.99 0.11 0.95 0.07 1.05 0.05 CALCIUM CHANNELS Nifedipine 1.06 0.17 0.95 0.12 1.03 0.09 CALCIUM CHANNELS Nimodipine 1.05 0.06 0.95 0.03 1.06 0.17 CALCIUM CHANNELS Nitrendipine 0.99 0.07 0.96 0.10 1.04 0.09 CALCIUM CHANNELS SDZ-202791 R(-) 1.01 0.08 0.92 0.06 1.01 0.08 CALCIUM CHANNELS SKF-96365 0.73 0.05 0.70 0.11 0.69 0.04 CALCIUM CHANNELS Tetrandrine 0.47 0.07 0.76 0.16 0.87 0.20 CALCIUM CHANNELS Verapamil 1.01 0.02 0.89 0.07 1.06 0.20 CALCIUM CHANNELS Methoxy Verapamil 0.93 0.14 0.96 0.07 0.93 0.13 CALCIUM CHANNELS Bepridil 0.70 0.16 0.92 0.15 0.84 0.14 CALCIUM CHANNELS Amiodarone 0.32 0.12 0.58 0.07 0.48 0.23 CALCIUM CHANNELS YS035 1.00 0.16 -

Application of Biomarker Compounds As Tracers for Sources and Fates of Natural and Anthropogenic Organic Matter in Tile Environment
AN ABSTRACT OF THE DISSERTATION OF Daniel R. Oros for the degree of Doctor of Philosophy in Environmental Sciences presented on September 24. 1999. Title: Application of Biomarker Compoundsas Tracers for Sources and Fates of Natural and Anthropogenic Organic Matter in the Environment. Redacted for Privacy Abstract approved: Bernd R.T. Simoneit Determination of the source and fate of natural (higher plant lipids, marine lipids, etc.) and anthropogenically (e.g., petroleum, coal emissions) derived hydrocarbons and oxygenated compounds in the environment was accomplished using gas chromatography (GC) and gas chromatography-mass spectrometry (GC- MS) to characterize or identify molecular biomarkers to be utilized as tracers. The distributions and abundances of biomarkers such as straight chain homologous series (e.g., n-alkanes, n-alkanoic acids, n-alkan-2-ones, n-alkanols, etc.) and cyclic terpenoid compounds (e.g., sesquiterpenoids, diterpenoids, steroids, triterpenoids) were identified in epicuticular waxes from conifers of western North America (natural emissions). These biomarkers and their thermal alteration derivativeswere also identified in smoke emissions from known vegetation sources (e.g., conifers, deciduous trees and grasses) and were then applied as tracers in soils, soils that contained wildfire residues and soillriver mud washout after wildfire burning. Where possible, the reaction pathways of transformation from the parentprecursor compounds to intermediate and final alteration products were determined from GC- MS data. In addition, molecular tracer analysis was applied to air, water and sediment samples collected from a lacustrine setting (Crater Lake, OR) in order to determine the identities, levels and fates of anthropogenic (i.e., petroleum hydrocarbon contamination from boating and related activities) hydrocarbons ina pristine organic matter sink. -

Interplay Between Gating and Block of Ligand-Gated Ion Channels
brain sciences Review Interplay between Gating and Block of Ligand-Gated Ion Channels Matthew B. Phillips 1,2, Aparna Nigam 1 and Jon W. Johnson 1,2,* 1 Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA; [email protected] (M.B.P.); [email protected] (A.N.) 2 Center for Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA * Correspondence: [email protected]; Tel.: +1-(412)-624-4295 Received: 27 October 2020; Accepted: 26 November 2020; Published: 1 December 2020 Abstract: Drugs that inhibit ion channel function by binding in the channel and preventing current flow, known as channel blockers, can be used as powerful tools for analysis of channel properties. Channel blockers are used to probe both the sophisticated structure and basic biophysical properties of ion channels. Gating, the mechanism that controls the opening and closing of ion channels, can be profoundly influenced by channel blocking drugs. Channel block and gating are reciprocally connected; gating controls access of channel blockers to their binding sites, and channel-blocking drugs can have profound and diverse effects on the rates of gating transitions and on the stability of channel open and closed states. This review synthesizes knowledge of the inherent intertwining of block and gating of excitatory ligand-gated ion channels, with a focus on the utility of channel blockers as analytic probes of ionotropic glutamate receptor channel function. Keywords: ligand-gated ion channel; channel block; channel gating; nicotinic acetylcholine receptor; ionotropic glutamate receptor; AMPA receptor; kainate receptor; NMDA receptor 1. Introduction Neuronal information processing depends on the distribution and properties of the ion channels found in neuronal membranes. -

Molecular Biology of Neuronal Voltage-Gated Calcium Channels
EXPERIMENTAL and MOLECULAR MEDICINE, Vol. 30, No 3, 123-130, September 1998 Molecular biology of neuronal voltage-gated calcium channels Hemin Chin and is capable of directing expression of calcium channel activity in heterologous expression systems. In the central Genetics Research Branch, Division of Basic and Clinical Neuroscience Research, nervous system (CNS), VGCCs are expressed by five National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland, distinct a1 subunit genes (α1A, α1B, α1C, α1D and α1E), U.S.A. which exhibit further variations due to alternative splicing of the primary RNA transcripts. The α1C and, α1D su b u n i t Accepted 3 August 1998 genes encode dihydropyridine (DHP)-sensitive L-type channels, while the three other α1 subunit genes (α1A, α1B and α1E) give rise to DHP-insensitive P/Q-, N- and R-type channels, respectively. The α2 and δ s u b u n i t proteins are produced by proteolytic cleavage of a larger precursor produced by the single α2-δ gene (Table 1). Introduction Three alternatively spliced variants of the α2 subunit are expressed in a tissue-specific manner. Two variants Calcium ions are important intracellular messengers have been isolated from the brain and skeletal muscle mediating a number of neuronal functions including neuro- (Kim et al., 1992; Williams et al., 1992), and a distinct transmitter release, neurosecretion, neuronal excitation, third splice variant which is expressed in glial cells has survival of eurons, and regulation of gene expression. been recently identified (Puro et al., 1996). In addition to The entry of calcium across the plasmamembrane in the gene encoding the skeletal muscle β subunit, three response to membrane depolarization or activation of 1 other β subunit genes (β2, β3 and β4) have been isolated neurotransmitter receptors represents a major pathway thus far. -

Title the Chemistry on Diterpenoids in 1965 Author(S)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kyoto University Research Information Repository Title The Chemistry on Diterpenoids in 1965 Author(s) Fujita, Eiichi Bulletin of the Institute for Chemical Research, Kyoto Citation University (1966), 44(3): 239-272 Issue Date 1966-10-31 URL http://hdl.handle.net/2433/76121 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University The Chemistry on Diterpenoids in 1965 Eiichi FuJITA* (FujitaLaboratory) ReceivedJune 20, 1966 I. INTRODUCTION Several reviews on diterpenoids have been published.*2 Last year, the author described the chemistry on diterpenoids in 1964 in outline.'7 The present review is concerned with the chemical works on diterpenoids in 1965. The classification consists of abietanes, pimaranes, labdanes, phyllocladanes, gibbanes, diterpene alkaloids, and the others. 1617 151613 2016 2©' 10gII 14 56.7 4®20 I20ei317115118 191 1819 14]5 18 19 AbietanePimaraneLabdane 20its1744a 13 4U15 36 15, 2.®7 110 ®® 18 199 8 PhyllocladaneGibbane II. ABIETANE AND ITS REOATED SKELETONS Lawrence et al.27 isolated palustric acid (2) from gum rosin. The selective crystallization of its 2,6-dimethylpiperidine salt, which precipitated from acetone solution of the rosin, from methanolacetone (1:1) was effective for isolation. The four conjugated dienic resin acids, namely, levopimaric (1), palustric (2), neoabietic (3), and abietic acid (4) were treated with an excess of potassium t- butoxide in dimethyl slufoxide solution at reflux temperature (189°) for 2 minutes.37 All four solutions then exhibited a single major peak in their U.V. spectra charac- teristic of abietic acid. -

Co-Assembly of N-Type Ca and BK Channels Underlies Functional
Research Article 985 Co-assembly of N-type Ca2+ and BK channels underlies functional coupling in rat brain David J. Loane*, Pedro A. Lima‡ and Neil V. Marrion§ Department of Pharmacology and MRC Centre for Synaptic Plasticity, University of Bristol, Bristol, BS8 1TD, UK *Present address: Laboratory for the Study of CNS Injury, Department of Neuroscience, Georgetown University Medical Center, Washington, DC 20057, USA ‡Present address: Dep. Fisiologia, Fac. Ciências Médicas, UNL, 1169-056 Lisboa, Portugal §Author for correspondence (e-mail: [email protected]) Accepted 9 January 2007 Journal of Cell Science 120, 985-995 Published by The Company of Biologists 2007 doi:10.1242/jcs.03399 Summary Activation of large conductance Ca2+-activated potassium and reproduced the interaction. Co-expression of (BK) channels hastens action potential repolarisation and CaV2.2/CaV3 subunits with Slo27 channels revealed rapid generates the fast afterhyperpolarisation in hippocampal functional coupling. By contrast, extremely rare examples pyramidal neurons. A rapid coupling of Ca2+ entry with of rapid functional coupling were observed with co- BK channel activation is necessary for this to occur, which expression of CaV1.2/CaV3 and Slo27 channels. Action might result from an identified coupling of Ca2+ entry potential repolarisation in hippocampal pyramidal neurons through N-type Ca2+ channels to BK channel activation. was slowed by the N-type channel blocker -conotoxin This selective coupling was extremely rapid and resistant GVIA, but not by the L-type channel blocker isradipine. to intracellular BAPTA, suggesting that the two channel These data showed that selective functional coupling types are close. -

Slow Inactivation in Voltage Gated Potassium Channels Is Insensitive to the Binding of Pore Occluding Peptide Toxins
Biophysical Journal Volume 89 August 2005 1009–1019 1009 Slow Inactivation in Voltage Gated Potassium Channels Is Insensitive to the Binding of Pore Occluding Peptide Toxins Carolina Oliva, Vivian Gonza´lez, and David Naranjo Centro de Neurociencias de Valparaı´so, Facultad de Ciencias, Universidad de Valparaı´so, Valparaı´so, Chile ABSTRACT Voltage gated potassium channels open and inactivate in response to changes of the voltage across the membrane. After removal of the fast N-type inactivation, voltage gated Shaker K-channels (Shaker-IR) are still able to inactivate through a poorly understood closure of the ion conduction pore. This, usually slower, inactivation shares with binding of pore occluding peptide toxin two important features: i), both are sensitive to the occupancy of the pore by permeant ions or tetraethylammonium, and ii), both are critically affected by point mutations in the external vestibule. Thus, mutual interference between these two processes is expected. To explore the extent of the conformational change involved in Shaker slow inactivation, we estimated the energetic impact of such interference. We used kÿconotoxin-PVIIA (kÿPVIIA) and charybdotoxin (CTX) peptides that occlude the pore of Shaker K-channels with a simple 1:1 stoichiometry and with kinetics 100-fold faster than that of slow inactivation. Because inactivation appears functionally different between outside-out patches and whole oocytes, we also compared the toxin effect on inactivation with these two techniques. Surprisingly, the rate of macroscopic inactivation and the rate of recovery, regardless of the technique used, were toxin insensitive. We also found that the fraction of inactivated channels at equilibrium remained unchanged at saturating kÿPVIIA. -

Glycine311, a Determinant of Paxilline Block in BK Channels: a Novel Bend in the BK S6 Helix Yu Zhou Washington University School of Medicine in St
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2010 Glycine311, a determinant of paxilline block in BK channels: A novel bend in the BK S6 helix Yu Zhou Washington University School of Medicine in St. Louis Qiong-Yao Tang Washington University School of Medicine in St. Louis Xiao-Ming Xia Washington University School of Medicine in St. Louis Christopher J. Lingle Washington University School of Medicine in St. Louis Follow this and additional works at: http://digitalcommons.wustl.edu/open_access_pubs Recommended Citation Zhou, Yu; Tang, Qiong-Yao; Xia, Xiao-Ming; and Lingle, Christopher J., ,"Glycine311, a determinant of paxilline block in BK channels: A novel bend in the BK S6 helix." Journal of General Physiology.135,5. 481-494. (2010). http://digitalcommons.wustl.edu/open_access_pubs/2878 This Open Access Publication is brought to you for free and open access by Digital Commons@Becker. It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. Published April 26, 2010 A r t i c l e Glycine311, a determinant of paxilline block in BK channels: a novel bend in the BK S6 helix Yu Zhou, Qiong-Yao Tang, Xiao-Ming Xia, and Christopher J. Lingle Department of Anesthesiology, Washington University School of Medicine, St. Louis, MO 63110 The tremorogenic fungal metabolite, paxilline, is widely used as a potent and relatively specific blocker of Ca2+- and voltage-activated Slo1 (or BK) K+ channels. The pH-regulated Slo3 K+ channel, a Slo1 homologue, is resistant to blockade by paxilline. -

An Advance About the Genetic Causes of Epilepsy
E3S Web of Conferences 271, 03068 (2021) https://doi.org/10.1051/e3sconf/202127103068 ICEPE 2021 An advance about the genetic causes of epilepsy Yu Sun1, a, *, †, Licheng Lu2, b, *, †, Lanxin Li3, c, *, †, Jingbo Wang4, d, *, † 1The School of Molecular and Cellular Biology, University of Illinois at Urbana-Champaign, Urbana, IL 61801-3633, US 2High School Affiliated to Shanghai Jiao Tong University, Shanghai, 200441, China 3Applied Biology program, University of British Columbia, Vancouver, V6r3b1, Canada 4School of Chemical Machinery and Safety, Dalian University of Technology, Dalian, 116023, China †These authors contributed equally. Abstract: Human hereditary epilepsy has been found related to ion channel mutations in voltage-gated channels (Na+, K+, Ca2+, Cl-), ligand gated channels (GABA receptors), and G-protein coupled receptors, such as Mass1. In addition, some transmembrane proteins or receptor genes, including PRRT2 and nAChR, and glucose transporter genes, such as GLUT1 and SLC2A1, are also about the onset of epilepsy. The discovery of these genetic defects has contributed greatly to our understanding of the pathology of epilepsy. This review focuses on introducing and summarizing epilepsy-associated genes and related findings in recent decades, pointing out related mutant genes that need to be further studied in the future. 1 Introduction Epilepsy is a neurological disorder characterized by 2 Malfunction of Ion channel epileptic seizures caused by abnormal brain activity. 1 in Functional variation in voltage or ligand-gated ion 100 (50 million people) people are affected by symptoms channel mutations is a major cause of idiopathic epilepsy, of this disorder worldwide, with men, young children, and especially in rare genetic forms.