Product Monograph
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Updatirg the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults
Updatirg the BeersCriteria for Potentially InappropriateMedication Use in Older Adults Resultsof a US ConsensusPanel of Experts DonnaM.Fich,PhD,RN;lamesW.Cooper,PhD,RPh;WilliamE.Wade,PhannD,FASHP,FCCP; JenniJerL. Waller, PhD;J, RossMaclean, MD; Marh H. Beers,MD Bcckground: Medication toxic effectsand drug- Reruhr: This study identified 48 individual medica- relatedproblems can have profound medical and safety tions or classeso[ medicationsto avoid in older adults consequencesfor older adults and economically affect the and their potential concernsand 20 diseases/conditions health caresystem. The purpose of this initiative was to and medicationsto be avoidedin older adultswith these reviseand update the Beerscriteria for potentially inap- conditions.Of thesepotentially inappropriate drugs, 66 propriate medicationuse in adults 65 yearsand older in wereconsidered by the panelto haveadverse outcomes the United States. of high severity. lYlcthcdr: This study used a modified Delphi method, a Concludonr: This study is an importantupdate of pre- setof proceduresand methodsfor formulating a groupjudg- viously establishedcriteria that have been widely used ment for a subject matter in which precise information is and cited. The application of the Beerscriteria and other Iacking. The criteria reviewed covered 2 types of state- tools for identifying potentially inapproprlate medica- ments: (l) medicationsor medicationclasses that should tion use will continue to enableproviders to plan inter- grnerally be avoidedin persons 65 years or older because -
![Pharmacological and Ionic Characterizations of the Muscarinic Receptors Modulating [3H]Acetylcholine Release from Rat Cortical Synaptosomes’](https://docslib.b-cdn.net/cover/3023/pharmacological-and-ionic-characterizations-of-the-muscarinic-receptors-modulating-3h-acetylcholine-release-from-rat-cortical-synaptosomes-753023.webp)
Pharmacological and Ionic Characterizations of the Muscarinic Receptors Modulating [3H]Acetylcholine Release from Rat Cortical Synaptosomes’
0270.6474/85/0505-1202$02.00/O The Journal of Neuroscience CopyrIght 0 Society for Neuroscrence Vol. 5, No. 5, pp. 1202-1207 Printed in U.S.A. May 1985 Pharmacological and Ionic Characterizations of the Muscarinic Receptors Modulating [3H]Acetylcholine Release from Rat Cortical Synaptosomes’ EDWIN M. MEYER* AND DEBORAH H. OTERO Department of Pharmacology and Therapeutics, University of Florida School of Medicine, Gainesville, Florida 32610 Abstract brain (Gonzales and Crews, 1984). M,-receptors, however, appear pre- and postsynaptically in brain, are regulated by an intrinsic The muscarinic receptors that modulate acetylcholine membrane protein that binds to GTP (g-protein), and may not be release from rat cortical synaptosomes were characterized coupled to changes in phosphatidylinositol turnover. with respect to sensitivity to drugs that act selectively at M, The present studies were designed to determine whether M,- or or Ma receptor subtypes, as well as to changes in ionic Mp-receptors mediate the presynaptic modulation of ACh release. strength and membrane potential. The modulatory receptors These studies involve dose-response curves for the release of appear to be of the M2 type, since they are activated by synaptosomal [3H]ACh in the presence of selected muscarinic ago- carbachol, acetylcholine, methacholine, oxotremorine, and nists and antagonists, as well as treatments that selectively alter MI- bethanechol, but not by pilocarpine, and are blocked by or M,-receptor activity. Our results indicate that the presynaptic atropine, scopolamine, and gallamine (at high concentra- modulation of [3H]ACh release is mediated by MP- but not MI- tions), but not by pirenzepine or dicyclomine. -

Supplemental Table 1: Patient Characteristics, Interventions and Definitions/Variables of Persistence, Adherence and Discontinuation Reported in the Studies
Adherence and persistence to OAB medication Supplemental Table 1: Patient characteristics, interventions and definitions/variables of persistence, adherence and discontinuation reported in the studies. Author (year) and Definition of persistence/adherence/discontinuation and Length of country (database) Participants Drug/intervention* independent variables on persistence follow-up Brostrøm and Hallas n=2477 Any prescription of OAB Patients who continued taking a particular drug for up to 7 years with Up to 7 (2009)1 Male: n=836 (33.8%) medication: no more than 120-day gaps were regarded as experiencing single- years Odense University Female: n=1641 (66.2%) flavoxate (n=21) treatment episodes Pharmacoepidemio- Mean age: 68.3 yearsa oxybutynin TD (n=48) logical Database tolterodine (n=1478) Variables: age, gender, prior use of OAB agents and use of (OPED); Denmark) solifenacin (n=774) anti-diabetic drugs (1999–2006) trospium (n=271) darifenacin (n=52) Chancellor et al (2013)2 n=103 250 First (new) prescription of OAB To be considered a discontinuation, patients were required to have a 2 years IMS Lifelink Database, Male: n≈25 916a (25.1%) medication in adults ≥18 years: gap of at least 45 days in therapy based on fill dates and days’ Connecticut; USA Female: n≈77 334a (74.9%) tolterodine ER (n=43 881)a supply (2005–2008) Mean (SD) age: 58.7 (15.7) solifenacin (n=15 488)a years oxybutynin (n=15 075)a Adherence rate was defined as the proportion of patients filling more darifenacin (n=10 532)a than one prescription with an MPR of ≥80% oxybutynin -

BETHANECHOL CHLORIDE TABLETS, USP5 Mg, 10 Mg, 25 Mg
BETHANECHOL CHLORIDE- bethanechol chloride tablet Upsher-Smith Laboratories, LLC ---------- BETHANECHOL CHLORIDE TABLETS, USP 5 mg, 10 mg, 25 mg and 50 mg Rx only DESCRIPTION Bethanechol chloride, USP, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related to acetylcholine. It is designated chemically as 2-[(aminocarbonyl)oxy]-N, N, N-trimethyl-1-propanaminium chloride. Its molecular formula is C7H17ClN2O2 and its structural formula is: It is a white, hygroscopic crystalline powder having a slight amine-like odor, freely soluble in water, and has a molecular weight of 196.68. Each tablet for oral administration contains 5 mg, 10 mg, 25 mg or 50 mg bethanechol chloride, USP. Tablets also contain the following inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, (25 mg and 50 mg) D&C Yellow #10 Lake and FD&C Yellow #6 Lake. CLINICAL PHARMACOLOGY Bethanechol chloride acts principally by producing the effects of stimulation of the parasympathetic nervous system. It increases the tone of the detrusor urinae muscle, usually producing a contraction sufficiently strong to initiate micturition and empty the bladder. It stimulates gastric motility, increases gastric tone and often restores impaired rhythmic peristalsis. Stimulation of the parasympathetic nervous system releases acetylcholine at the nerve endings. When spontaneous stimulation is reduced and therapeutic intervention is required, acetylcholine can be given, but it is rapidly hydrolyzed by cholinesterase and its effects are transient. Bethanechol chloride is not destroyed by cholinesterase and its effects are more prolonged than those of acetylcholine. Effects on the GI and urinary tracts sometimes appear within 30 minutes after oral administration of bethanechol chloride, but more often 60 to 90 minutes are required to reach maximum effectiveness. -
Role of Muscarinic Cholinergic Receptors in Regulation Of
Proc. Nat. Acad. Sci. USA Vol. 69, No. 11, pp. 3287-3291, November 1972 Role of Muscarinic Cholinergic Receptors in Regulation of Guanosine 3':5'-Cyclic Monophosphate Content in Mammalian Brain, Heart Muscle, and Intestinal Smooth Muscle (cyclic AMP/nicotine receptors/atropine) TEE-PING LEE, J. F. KUO, AND PAUL GREENGARD* Department of Pharmacology, Yale University School of Medicine, 333 Cedar St., New Haven, Connecticut 06510 Communicated by Lewis Thomas, August 22, 1972 ABSTRACT Effects of acetylcholine and of agents that Recent studies indicate that acetylcholine can cause the mimic or block its physiological actions have been studied upon concentrations of guanosine 3':5'-cyclic monophos- accumulation of cyclic GMP in heart (10, 11), brain (11, 12), phate (cyclic GMP) and adenosine 3':5'-cyclic monophos- and ductus deferens (13). Perfusion of isolated rat heart with phate (cyclic AMP) in slices of mammalian cerebral cortex, acetylcholine caused an increase of up to 140% in cyclic heart ventricle, and ileum. Acetylcholine, and cholino- GMP content (10). Moreover, application of low concentra- mimetic agents with a predominantly muscarinic action, tions of acetylcholine to slices of rat heart caused increases of such as methacholine, bethanechol, and pilocarpine, induced an increase in the concentration of cyclic GMP, up to 10-fold in the concentration of cyclic GMP (11). The accompanied by no change or a slight decrease in the con- amount of cyclic GMP in brains of rats was increased up to centration of cyclic AMP, in all three tissues studied. 80% (12) by the injection of oxotremorine, an agent known to Tetramethylammonium, a cholinomimetic agent with a affect cholinergic mechanisms in the nervous system. -

August 2021 California Signaturevalue 4 Tier HMO Formulary
Pharmacy | Formulary | California 2021 California SignatureValue 4-Tier HMO Formulary Please note: This Formulary is accurate as of August 1, 2021 and is subject to change after this date. All previous versions of this Formulary are no longer in effect. Your estimated coverage and copay/coinsurance may vary based on the benefit plan you choose and the effective date of the plan. This Formulary can also be accessed online at myuhc.com > Pharmacy Information > Prescription Drug Lists > California plans > SignatureValue HMO plans. Plan-specific coverage documents may be accessed online at uhc.com/statedruglists > Small Group Plans > California. If you are a UnitedHealthcare member, please register or log on to myuhc.com, or call the toll-free number on your health plan ID card to find pharmacy information specific to your benefit plan. This Formulary is applicable to the following health insurance products offered by UnitedHealthcare: • SignatureValue • SignatureValue Advantage • SignatureValue Alliance • SignatureValue Flex • SignatureValue Focus • SignatureValue Harmony • SignatureValue Performance Updated 6/17/2021 6/21 © 2021 United HealthCare Services, Inc. All Rights Reserved. WF3890815-N Contents At UnitedHealthcare, we want to help you better understand your medication options. ..................................................... 3 How do I use my Formulary? ............................................. 4 What are tiers? ........................................................ 5 When does the Formulary change? ........................................ 5 Utilization Management Programs ......................................... 6 Your Right to Request Access to a Non-formulary Drug ....................... 6 Requesting a Prior Authorization or Step Therapy Exception ................... 7 How do I locate and fill a prescription through a retail network pharmacy? . 7 How do I locate and fill a prescription through the mail order pharmacy? . 7 How do I locate and fill a prescription at a specialty pharmacy? ............... -

Drug-Facilitated Sexual Assault in the U.S
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Estimate of the Incidence of Drug-Facilitated Sexual Assault in the U.S. Document No.: 212000 Date Received: November 2005 Award Number: 2000-RB-CX-K003 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant final report available electronically in addition to traditional paper copies. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. AWARD NUMBER 2000-RB-CX-K003 ESTIMATE OF THE INCIDENCE OF DRUG-FACILITATED SEXUAL ASSAULT IN THE U.S. FINAL REPORT Report prepared by: Adam Negrusz, Ph.D. Matthew Juhascik, Ph.D. R.E. Gaensslen, Ph.D. Draft report: March 23, 2005 Final report: June 2, 2005 Forensic Sciences Department of Biopharmaceutical Sciences (M/C 865) College of Pharmacy University of Illinois at Chicago 833 South Wood Street Chicago, IL 60612 ABSTRACT The term drug-facilitated sexual assault (DFSA) has been recently coined to describe victims who were given a drug by an assailant and subsequently sexually assaulted. Previous studies that have attempted to determine the prevalence of drugs in sexual assault complainants have had serious biases. This research was designed to better estimate the rate of DFSA and to examine the social aspects surrounding it. Four clinics were provided with sexual assault kits and asked to enroll sexual assault complainants. -

Effects of YM905, a Novel Muscarinic M3-Receptor Antagonist, on Experimental Models of Bowel Dysfunction in Vivo
Jpn. J. Pharmacol. 86, 281 – 288 (2001) Effects of YM905, a Novel Muscarinic M3-Receptor Antagonist, on Experimental Models of Bowel Dysfunction In Vivo Seiji Kobayashi*, Ken Ikeda, Mami Suzuki, Toshimitsu Yamada and Keiji Miyata Pharmacology Laboratories, Institute for Drug Discovery Research, Yamanouchi Pharmaceutical Co., Ltd., 21 Miyukigaoka, Tsukuba-shi, Ibaraki 305-8585, Japan Received January 24, 2001 Accepted April 2, 2001 ABSTRACT—We investigated the effects of YM905 [(+)-(1S,3'R)-quinuclidin-3'-yl 1-phenyl-1,2,3,4-tetra- hydroisoquinoline-2-carboxylate monosuccinate], a new orally active muscarinic M3-receptor antagonist, on bowel dysfunction in vivo using experimental models that reproduce the symptoms present in irritable bowel syndrome (IBS). YM905 potently inhibited restraint stress-induced fecal pellet output in fed rats (ED50: 4.0 mg/kg) and diarrhea in fasted rats (ED50: 1.7 mg/kg), with similar potencies to the inhibition of bethanechol-, neostigmine- and nicotine-induced fecal pellet output in rats (ED50: 3.3, 7.9 and 4.5 mg/kg, respectively). YM905 also inhibited 5-hydroxytryptamine (5-HT)-, prostaglandin E2- and castor oil-induced secretory diarrhea in mice (ED50: 5.5, 14 and 6.3 mg/kg, respectively), but showed no significant effect on cholera toxin-induced intestinal secretion in mice. In addition, YM905 (3, 10 mg/kg) reversed morphine- decreased postprandial defecation in ferrets, a model of spastic constipation, whereas remosetron, a 5-HT3- receptor antagonist, was not effective. The mode of YM905 action was similar to that of darifenacin, a selec- tive M3-receptor antagonist, with equivalent potencies. By contrast, propantheline, an antimuscarinic drug that has been used for IBS, was much less potent. -

Stress Urinary Incontinence: a Closer Look at Nonsurgical Therapies
OBGMANAGEMENT ■ OBG BY PETER K. SAND, MD; G. WILLY DAVILA, MD; KARL LUBER, MD; and DEBORAH L. MYERS, MD Stress urinary incontinence: A closer look at nonsurgical therapies This pervasive condition has spawned a host of treatments, from conservative measures like pelvic floor rehabilitation to cutting-edge modalities such as radiofrequency therapy. In this discussion, a panel of experts compares the less invasive options and offers pearls on evaluating and counseling patients and selecting appropriate treatments. e know what it is: The involuntary tried and true and brand new? To address loss of urine during activities that this question, OBG MANAGEMENT convened Wincrease intra-abdominal pressure. a panel of expert urogynecologists. Their And we know what it isn’t: Rare. We even focus was conservative therapies. know how to treat stress incontinence, since it affects women of all ages and generally can be A wide range of options attributed to urethral hypermobility and/or to preserve fertility intrinsic sphincter deficiency. But are we up- SAND: We are fortunate to have a number of to-date on all the management options, both very effective nonsurgical treatments for stress urinary incontinence (SUI). Why don’t we begin by laying out the full complement O UR PANELISTS of options? Dr. Davila, when a pre- ■ Peter K. Sand, MD, moderator of this menopausal woman presents to your center discussion, is professor of obstetrics and with SUI, what therapies do you offer if she gynecology at the Feinberg School of Medicine, is waiting to complete childbearing? And Northwestern University, Evanston, Ill. how do you counsel her? DAVILA: ■ G. -

(Pelvic Floor Muscles Training) and Drug Plus Biofeedback on Quality
Obstet Gynecol Cancer Res. 2016 November; 1(3):e8678. doi: 10.17795/ojcr-8678. Published online 2016 November 27. Research Article Comparing the Effect of Tolterodine, Biofeedback (Pelvic Floor Muscles Training) and Drug plus Biofeedback on Quality of Life and Urge Urinary Incontinency in Patients Referred to Imam Khomeini Hospital in 2014 Maryam Deldar Pasikhani,1 Zinat Ghanbari,1 Fateme Talei Khatibi,2,* Ali Ganjalikhan Hakemi,3 and Elaheh Miri Ashtiani4 1Department of Obstetrics and Gynecology, Imam Khomeini Hospital, Tehran, Iran 2Department of Obstetrics and Gynecology, Valiasr Hospital, Tehran University of Medical Sciences, Tehran, Iran 3Valiasr Hospital, Tehran University of Medical Sciences, Tehran, Iran 4Department of Physiotherapy, Faculty of Rehabilitation Sciences, Shahid Beheshti University of Medical Sciences, Damavand, Tehran, Iran *Corresponding author: Fateme Talei Khatibi, Department of Obstetrics and Gynecology, Valiasr Hospital, Tehran University of Medical Sciences, Tehran, Iran. E-mail: [email protected] Received 2016 September 06; Revised 2016 November 09; Accepted 2016 November 21. Abstract Background: Urgency is a characteristic for overactive bladder and is defined by a sudden obligatory need for urination, a feeling that can be hardly stopped. Many methods such as drug therapy and feedback have been used to treat urinary incontinency. Objectives: The aim of this study was to assess and compare the effect of medication, biofeedback or biofeedback plus medication on urge- urinary incontinency and quality of life of patients. Methods: This was a case-control randomized clinical trial performed on patients referred to Imam Khomeini hospital in 2014. Pa- tients were divided into three groups of drug (Tolterodine), biofeedback, and biofeedback plus drug. -
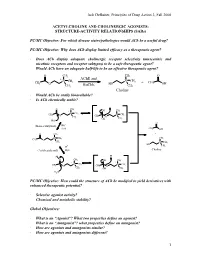
Jack Deruiter, Principles of Drug Action 2, Fall 2000 1 ACETYLCHOLINE and CHOLINERGIC AGONISTS: STRUCTURE-ACTIVITY RELATIONSHIPS
Jack DeRuiter, Principles of Drug Action 2, Fall 2000 ACETYLCHOLINE AND CHOLINERGIC AGONISTS: STRUCTURE-ACTIVITY RELATIONSHIPS (SARs) PC/MC Objective: For which disease states/pathologies would ACh be a useful drug? PC/MC Objective: Why does ACh display limited efficacy as a therapeutic agent? · Does ACh display adequate cholinergic receptor selectivity (muscarinic and nicotinic receptors and receptor subtypes) to be a safe therapeutic agent? · Would ACh have an adequate half-life to be an effective therapeutic agent? O CH3 CH3 O + AChE and + CH CH N CH3 N 3 + CH 3 O BuChE HO 3 OH CH3 CH3 Choline · Would ACh be orally bioavailable? · Is ACh chemically stable? O CH3 + OH CH3 + N CH3 CH3 N CH3 O CH3 CH3 O OH CH3 HO- O - (Base-catalyzed) HO H2O CH3 OH O CH3 + + N CH CH CH 3 3 3 O + CH3 N CH3 HO H+ CH3 (Acid-catalyzed) H2O Choline + H+ H O CH3 OH CH3 + + N CH3 N CH3 CH3 O CH3 CH O 3 OH CH3 H2O PC/MC Objective: How could the structure of ACh be modified to yield derivatives with enhanced therapeutic potential? · Selective agonist activity? · Chemical and metabolic stability? Global Objectives: · What is an “Agonist”? What two properties define an agonist? · What is an “Antagonist”? what properties define an antagonist? · How are agonists and antagonists similar? · How are agonists and antagonists different? 1 Jack DeRuiter, Principles of Drug Action 2, Fall 2000 MC Objective: Which ACh structural features appear to be required or at least contribute to cholinergic receptor binding. O CH3 + N CH3 CH3 O CH3 Ester Quaternary Ammonium MC Objective: