EAU Guidelines on Neuro-Urology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Chronic Pelvic Pain M
Guidelines on Chronic Pelvic Pain M. Fall (chair), A.P. Baranowski, S. Elneil, D. Engeler, J. Hughes, E.J. Messelink, F. Oberpenning, A.C. de C. Williams © European Association of Urology 2008 TABLE OF CONTENTS PAGE 1. INTRODUCTION 5 1.1 The guideline 5 1.1.1 Publication history 5 1.2 Level of evidence and grade recommendations 5 1.3 References 6 1.4 Definition of pain (World Health Organization [WHO]) 6 1.4.1 Innervation of the urogenital system 7 1.4.2 References 8 1.5 Pain evaluation and measurement 8 1.5.1 Pain evaluation 8 1.5.2 Pain measurement 8 1.5.3 References 9 2. CHRONIC PELVIC PAIN 9 2.1 Background 9 2.1.1 Introduction to urogenital pain syndromes 9 2.2 Definitions of chronic pelvic pain and terminology (Table 4) 11 2.3 Classification of chronic pelvic pain syndromes 12 Table 3: EAU classification of chronic urogenital pain syndromes (page 10) Table 4: Definitions of chronic pain terminology (page 11) Table 5: ESSIC classification of types of bladder pain syndrome according to the results of cystoscopy with hydrodistension and of biopsies (page 13) 2.4 References 13 2.5 An algorithm for chronic pelvic pain diagnosis and treatment 13 2.5.1 How to use the algorithm 13 2.6 Prostate pain syndrome (PPS) 15 2.6.1 Introduction 16 2.6.2 Definition 16 2.6.3 Pathogenesis 16 2.6.4 Diagnosis 17 2.6.5 Treatment 17 2.6.5.1 Alpha-blockers 17 2.6.5.2 Antibiotic therapy 17 2.6.5.3 Non-steroidal anti-inflammatory drugs (NSAIDs) 17 2.6.5.4 Corticosteroids 17 2.6.5.5 Opioids 17 2.6.5.6 5-alpha-reductase inhibitors 18 2.6.5.7 Allopurinol 18 2.6.5.8 -

Updatirg the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults
Updatirg the BeersCriteria for Potentially InappropriateMedication Use in Older Adults Resultsof a US ConsensusPanel of Experts DonnaM.Fich,PhD,RN;lamesW.Cooper,PhD,RPh;WilliamE.Wade,PhannD,FASHP,FCCP; JenniJerL. Waller, PhD;J, RossMaclean, MD; Marh H. Beers,MD Bcckground: Medication toxic effectsand drug- Reruhr: This study identified 48 individual medica- relatedproblems can have profound medical and safety tions or classeso[ medicationsto avoid in older adults consequencesfor older adults and economically affect the and their potential concernsand 20 diseases/conditions health caresystem. The purpose of this initiative was to and medicationsto be avoidedin older adultswith these reviseand update the Beerscriteria for potentially inap- conditions.Of thesepotentially inappropriate drugs, 66 propriate medicationuse in adults 65 yearsand older in wereconsidered by the panelto haveadverse outcomes the United States. of high severity. lYlcthcdr: This study used a modified Delphi method, a Concludonr: This study is an importantupdate of pre- setof proceduresand methodsfor formulating a groupjudg- viously establishedcriteria that have been widely used ment for a subject matter in which precise information is and cited. The application of the Beerscriteria and other Iacking. The criteria reviewed covered 2 types of state- tools for identifying potentially inapproprlate medica- ments: (l) medicationsor medicationclasses that should tion use will continue to enableproviders to plan inter- grnerally be avoidedin persons 65 years or older because -

Acute Onset Flank Pain-Suspicion of Stone Disease (Urolithiasis)
Date of origin: 1995 Last review date: 2015 American College of Radiology ® ACR Appropriateness Criteria Clinical Condition: Acute Onset Flank Pain—Suspicion of Stone Disease (Urolithiasis) Variant 1: Suspicion of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 8 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). US color Doppler kidneys and bladder 6 O retroperitoneal Radiography intravenous urography 4 ☢☢☢ MRI abdomen and pelvis without IV 4 MR urography. O contrast MRI abdomen and pelvis without and with 4 MR urography. O IV contrast This procedure can be performed with US X-ray abdomen and pelvis (KUB) 3 as an alternative to NCCT. ☢☢ CT abdomen and pelvis with IV contrast 2 ☢☢☢ *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Recurrent symptoms of stone disease. Radiologic Procedure Rating Comments RRL* CT abdomen and pelvis without IV 7 Reduced-dose techniques are preferred. contrast ☢☢☢ This procedure is indicated in an emergent setting for acute management to evaluate for hydronephrosis. For planning and US color Doppler kidneys and bladder 7 intervention, US is generally not adequate O retroperitoneal and CT is complementary as CT more accurately characterizes stone size and location. This procedure is indicated if CT without contrast does not explain pain or reveals CT abdomen and pelvis without and with 6 an abnormality that should be further IV contrast ☢☢☢☢ assessed with contrast (eg, stone versus phleboliths). -
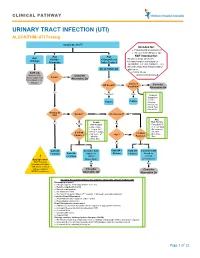
URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing
CLINICAL PATHWAY URINARY TRACT INFECTION (UTI) ALGORITHM- UTI Testing Suspicion of UTI Intended for: • Patients with presumed UTI • Greater than 60days of age Age Age NOT intended for: Age 60days- >36months or • Known urologic anomalies <60days • 36months Toilet Trained Chronic/complex conditions (ie. spinabifida, self cath, hardware, etc.) • Recent urinary tract instrumentation Clean Catch UA placement Cath UA • Critical Illness Refer to CCG Consider • Immunocompromised Fever? NO “Fever, infant (less Alternative Dx than 28days or 28- 90days)” Index of UA Result? Neg Low Consider Suspicion* Alternative Dx Yes Pos/Equiv High *Index of Suspicion • Febrile Culture Culture • Dysuria • Frequency • Flank Pain • Hx of UTI History of No Gender? Male Circumcised? YES UTI? Male Female Risk Factors Yes NO Female Risk Factors • Temp ≥ 39°C • Age <12mo • Fever ≥2 days • Temp ≥ 39°C • No source of • ≥ 3 Risk Fever ≥ 2 days infection • No source of ≥ 3 Risk • Non-black Factors? <1yr? No infection Factors? Race • White Race Yes Yes No Yes No Cath UA Consider Cath Cath UA + Cath UA Consider Cath + Culture Cath UA based on Culture + Culture based on ! + Culture clinical clinical Bag Specimen presentation presentation NOT Preferred Neg Neg (consider with labial adhesions, or failed catheterizations) Consider Consider NEVER send culture Alternative Dx Alternative Dx Imaging Recommendations for patients >2months after 1st Febrile UTI No imaging required o Prompt response to therapy (afebrile in 72 hrs) o Reliable outpatient follow up o Normal voiding pattern -

Impact of Urolithiasis and Hydronephrosis on Acute Kidney Injury in Patients with Urinary Tract Infection
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Impact of urolithiasis and hydronephrosis on acute kidney injury in patients with urinary tract infection Short title: Impact of urolithiasis and hydronephrosis on AKI in UTI Chih-Yen Hsiao1,2, Tsung-Hsien Chen1, Yi-Chien Lee3,4, Ming-Cheng Wang5,* 1Division of Nephrology, Department of Internal Medicine, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chia-Yi, Taiwan 2Department of Hospital and Health Care Administration, Chia Nan University of Pharmacy and Science, Tainan, Taiwan 3Department of Internal Medicine, Fu Jen Catholic University Hospital, Fu Jen Catholic University, New Taipei, Taiwan 4School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei, Taiwan 5Division of Nephrology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan *[email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.07.13.200337; this version posted July 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Background: Urolithiasis is a common cause of urinary tract obstruction and urinary tract infection (UTI). This study aimed to identify whether urolithiasis with or without hydronephrosis has an impact on acute kidney injury (AKI) in patients with UTI. -

A Review of the Effects of Pain and Analgesia on Immune System Function and Inflammation: Relevance for Preclinical Studies
Comparative Medicine Vol 69, No 6 Copyright 2019 December 2019 by the American Association for Laboratory Animal Science Pages 520–534 Overview A Review of the Effects of Pain and Analgesia on Immune System Function and Inflammation: Relevance for Preclinical Studies George J DeMarco1* and Elizabeth A Nunamaker2 One of the most significant challenges facing investigators, laboratory animal veterinarians, and IACUCs, is how to bal- ance appropriate analgesic use, animal welfare, and analgesic impact on experimental results. This is particularly true for in vivo studies on immune system function and inflammatory disease. Often times the effects of analgesic drugs on a particu- lar immune function or model are incomplete or don’t exist. Further complicating the picture is evidence of the very tight integration and bidirectional functionality between the immune system and branches of the nervous system involved in nociception and pain. These relationships have advanced the concept of understanding pain as a protective neuroimmune function and recognizing pathologic pain as a neuroimmune disease. This review strives to summarize extant literature on the effects of pain and analgesia on immune system function and inflammation in the context of preclinical in vivo studies. The authors hope this work will help to guide selection of analgesics for preclinical studies of inflammatory disease and immune system function. Abbreviations and acronyms: CB,Endocannabinoid receptor; CD,Crohn disease; CFA, Complete Freund adjuvant; CGRP,Calcitonin gene-related -

Purinergic P2 Receptors As Targets for Novel Analgesics
Pharmacology & Therapeutics 110 (2006) 433 – 454 www.elsevier.com/locate/pharmthera Purinergic P2 receptors as targets for novel analgesics Geoffrey Burnstock * Autonomic Neuroscience Centre, Royal Free and University College Medical School, Rowland Hill Street, London NW3 2PF, UK Abstract Following hints in the early literature about adenosine 5V-triphosphate (ATP) injections producing pain, an ion-channel nucleotide receptor was cloned in 1995, P2X3 subtype, which was shown to be localized predominantly on small nociceptive sensory nerves. Since then, there has been an increasing number of papers exploring the role of P2X3 homomultimer and P2X2/3 heteromultimer receptors on sensory nerves in a wide range of organs, including skin, tongue, tooth pulp, intestine, bladder, and ureter that mediate the initiation of pain. Purinergic mechanosensory transduction has been proposed for visceral pain, where ATP released from epithelial cells lining the bladder, ureter, and intestine during distension acts on P2X3 and P2X2/3, and possibly P2Y, receptors on subepithelial sensory nerve fibers to send messages to the pain centers in the brain as well as initiating local reflexes. P1, P2X, and P2Y receptors also appear to be involved in nociceptive neural pathways in the spinal cord. P2X4 receptors on spinal microglia have been implicated in allodynia. The involvement of purinergic signaling in long-term neuropathic pain and inflammation as well as acute pain is discussed as well as the development of P2 receptor antagonists as novel analgesics. D -

Obstructive Nephropathy Saulo Klahr
REVIEW ARTICLE Obstructive Nephropathy Saulo Klahr Abstract ages. The incidence of hydronephrosis reported by Bell (1) in a series of32,360 autopsies was 3.8% (3.9% in males, 3.6% in Obstructive nephropathy is a relatively commonentity females). The incidence of clinical manifestations of obstruc- that is treatable and often reversible. It occurs at all ages tive uropathy prior to death was not reported, and it is likely from infancy to elderly subjects. Obstructive uropathy is that hydronephrosis was an incidental finding in many of these classified according to the degree, duration and site of the patients. The incidence of hydronephrosis at autopsy is some- obstruction. It is the result of functional or anatomic le- what lower in children than in adults, being 2%in one series of sions located in the urinary tract. The causes of obstructive 16, 100 autopsies (2). Over 80% of children with hydronephro- uropathy are many. Obstruction of the urinary tract may sis at autopsy were less than 1 year old, with the balance of decrease renal blood flow and the glomerular filtration rate. childhood cases being distributed uniformly through the child- Several abnormalities in tubular function mayoccur in hood years. About 166 patients per 100,000 population had a obstructive nephropathy. These include decreased reab- presumptive diagnosis of obstructive uropathy on admission sorption of solutes and water, inability to concentrate the to hospitals in the United States in 1985 (3). Amongmale pa- urine and impaired excretion of hydrogen and potassium. tients with kidney and urologic disorders, obstructive uropa- Renal interstitial fibrosis is a commonfinding in patients thy ranked fourth at discharge (242 patients/100,000 dis- with long-term obstructive uropathy. -
![Pharmacological and Ionic Characterizations of the Muscarinic Receptors Modulating [3H]Acetylcholine Release from Rat Cortical Synaptosomes’](https://docslib.b-cdn.net/cover/3023/pharmacological-and-ionic-characterizations-of-the-muscarinic-receptors-modulating-3h-acetylcholine-release-from-rat-cortical-synaptosomes-753023.webp)
Pharmacological and Ionic Characterizations of the Muscarinic Receptors Modulating [3H]Acetylcholine Release from Rat Cortical Synaptosomes’
0270.6474/85/0505-1202$02.00/O The Journal of Neuroscience CopyrIght 0 Society for Neuroscrence Vol. 5, No. 5, pp. 1202-1207 Printed in U.S.A. May 1985 Pharmacological and Ionic Characterizations of the Muscarinic Receptors Modulating [3H]Acetylcholine Release from Rat Cortical Synaptosomes’ EDWIN M. MEYER* AND DEBORAH H. OTERO Department of Pharmacology and Therapeutics, University of Florida School of Medicine, Gainesville, Florida 32610 Abstract brain (Gonzales and Crews, 1984). M,-receptors, however, appear pre- and postsynaptically in brain, are regulated by an intrinsic The muscarinic receptors that modulate acetylcholine membrane protein that binds to GTP (g-protein), and may not be release from rat cortical synaptosomes were characterized coupled to changes in phosphatidylinositol turnover. with respect to sensitivity to drugs that act selectively at M, The present studies were designed to determine whether M,- or or Ma receptor subtypes, as well as to changes in ionic Mp-receptors mediate the presynaptic modulation of ACh release. strength and membrane potential. The modulatory receptors These studies involve dose-response curves for the release of appear to be of the M2 type, since they are activated by synaptosomal [3H]ACh in the presence of selected muscarinic ago- carbachol, acetylcholine, methacholine, oxotremorine, and nists and antagonists, as well as treatments that selectively alter MI- bethanechol, but not by pilocarpine, and are blocked by or M,-receptor activity. Our results indicate that the presynaptic atropine, scopolamine, and gallamine (at high concentra- modulation of [3H]ACh release is mediated by MP- but not MI- tions), but not by pirenzepine or dicyclomine. -

Phytochem Referenzsubstanzen
High pure reference substances Phytochem Hochreine Standardsubstanzen for research and quality für Forschung und management Referenzsubstanzen Qualitätssicherung Nummer Name Synonym CAS FW Formel Literatur 01.286. ABIETIC ACID Sylvic acid [514-10-3] 302.46 C20H30O2 01.030. L-ABRINE N-a-Methyl-L-tryptophan [526-31-8] 218.26 C12H14N2O2 Merck Index 11,5 01.031. (+)-ABSCISIC ACID [21293-29-8] 264.33 C15H20O4 Merck Index 11,6 01.032. (+/-)-ABSCISIC ACID ABA; Dormin [14375-45-2] 264.33 C15H20O4 Merck Index 11,6 01.002. ABSINTHIN Absinthiin, Absynthin [1362-42-1] 496,64 C30H40O6 Merck Index 12,8 01.033. ACACETIN 5,7-Dihydroxy-4'-methoxyflavone; Linarigenin [480-44-4] 284.28 C16H12O5 Merck Index 11,9 01.287. ACACETIN Apigenin-4´methylester [480-44-4] 284.28 C16H12O5 01.034. ACACETIN-7-NEOHESPERIDOSIDE Fortunellin [20633-93-6] 610.60 C28H32O14 01.035. ACACETIN-7-RUTINOSIDE Linarin [480-36-4] 592.57 C28H32O14 Merck Index 11,5376 01.036. 2-ACETAMIDO-2-DEOXY-1,3,4,6-TETRA-O- a-D-Glucosamine pentaacetate 389.37 C16H23NO10 ACETYL-a-D-GLUCOPYRANOSE 01.037. 2-ACETAMIDO-2-DEOXY-1,3,4,6-TETRA-O- b-D-Glucosamine pentaacetate [7772-79-4] 389.37 C16H23NO10 ACETYL-b-D-GLUCOPYRANOSE> 01.038. 2-ACETAMIDO-2-DEOXY-3,4,6-TRI-O-ACETYL- Acetochloro-a-D-glucosamine [3068-34-6] 365.77 C14H20ClNO8 a-D-GLUCOPYRANOSYLCHLORIDE - 1 - High pure reference substances Phytochem Hochreine Standardsubstanzen for research and quality für Forschung und management Referenzsubstanzen Qualitätssicherung Nummer Name Synonym CAS FW Formel Literatur 01.039. -

Trpa1) Activity by Cdk5
MODULATION OF TRANSIENT RECEPTOR POTENTIAL CATION CHANNEL, SUBFAMILY A, MEMBER 1 (TRPA1) ACTIVITY BY CDK5 A dissertation submitted to Kent State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy by Michael A. Sulak December 2011 Dissertation written by Michael A. Sulak B.S., Cleveland State University, 2002 Ph.D., Kent State University, 2011 Approved by _________________, Chair, Doctoral Dissertation Committee Dr. Derek S. Damron _________________, Member, Doctoral Dissertation Committee Dr. Robert V. Dorman _________________, Member, Doctoral Dissertation Committee Dr. Ernest J. Freeman _________________, Member, Doctoral Dissertation Committee Dr. Ian N. Bratz _________________, Graduate Faculty Representative Dr. Bansidhar Datta Accepted by _________________, Director, School of Biomedical Sciences Dr. Robert V. Dorman _________________, Dean, College of Arts and Sciences Dr. John R. D. Stalvey ii TABLE OF CONTENTS LIST OF FIGURES ............................................................................................... iv LIST OF TABLES ............................................................................................... vi DEDICATION ...................................................................................................... vii ACKNOWLEDGEMENTS .................................................................................. viii CHAPTER 1: Introduction .................................................................................. 1 Hypothesis and Project Rationale