Enterococci, from Harmless Bacteria to a Pathogen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Current Trends of Enterococci in Dairy Products: a Comprehensive Review of Their Multiple Roles
foods Review Current Trends of Enterococci in Dairy Products: A Comprehensive Review of Their Multiple Roles Maria de Lurdes Enes Dapkevicius 1,2,* , Bruna Sgardioli 1,2 , Sandra P. A. Câmara 1,2, Patrícia Poeta 3,4 and Francisco Xavier Malcata 5,6,* 1 Faculty of Agricultural and Environmental Sciences, University of the Azores, 9700-042 Angra do Heroísmo, Portugal; [email protected] (B.S.); [email protected] (S.P.A.C.) 2 Institute of Agricultural and Environmental Research and Technology (IITAA), University of the Azores, 9700-042 Angra do Heroísmo, Portugal 3 Microbiology and Antibiotic Resistance Team (MicroART), Department of Veterinary Sciences, University of Trás-os-Montes and Alto Douro (UTAD), 5001-801 Vila Real, Portugal; [email protected] 4 Associated Laboratory for Green Chemistry (LAQV-REQUIMTE), University NOVA of Lisboa, 2829-516 Lisboa, Portugal 5 LEPABE—Laboratory for Process Engineering, Environment, Biotechnology and Energy, Faculty of Engineering, University of Porto, 420-465 Porto, Portugal 6 FEUP—Faculty of Engineering, University of Porto, 4200-465 Porto, Portugal * Correspondence: [email protected] (M.d.L.E.D.); [email protected] (F.X.M.) Abstract: As a genus that has evolved for resistance against adverse environmental factors and that readily exchanges genetic elements, enterococci are well adapted to the cheese environment and may reach high numbers in artisanal cheeses. Their metabolites impact cheese flavor, texture, Citation: Dapkevicius, M.d.L.E.; and rheological properties, thus contributing to the development of its typical sensorial properties. Sgardioli, B.; Câmara, S.P.A.; Poeta, P.; Due to their antimicrobial activity, enterococci modulate the cheese microbiota, stimulate autoly- Malcata, F.X. -

Phage Infection Mediates Inhibition of Bystander Bacteria
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.11.077669; this version posted June 12, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Phage infection mediates inhibition of bystander bacteria 2 3 Anushila Chatterjeea*, Julia L. E. Willettb*, Gary M. Dunnyb, Breck A. Duerkopa,# 4 5 aDepartment of Immunology and Microbiology, University of Colorado School of Medicine, Aurora, 6 CO, USA, 80045. bDepartment of Microbiology and Immunology, University of Minnesota Medical 7 School, Minneapolis, MN, USA, 55455. 8 9 #Correspondence: Breck A. Duerkop [email protected] 10 *A.C. and J.L.E.W. contributed equally to this work. 11 12 13 Running title: Phage induced T7SS promotes antibacterial antagonism 14 15 Key words: bacteriophages, Enterococcus, antibiotic resistance, phage–bacteria interactions, 16 bacterial secretion systems, type VII secretion, contact-dependent antagonism 17 18 19 20 21 22 23 24 25 26 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.05.11.077669; this version posted June 12, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 27 Abstract 28 Bacteriophages (phages) are being considered as alternative therapeutics for the treatment 29 of multidrug resistant bacterial infections. -

Identification of Clinically Relevant Streptococcus and Enterococcus
pathogens Article Identification of Clinically Relevant Streptococcus and Enterococcus Species Based on Biochemical Methods and 16S rRNA, sodA, tuf, rpoB, and recA Gene Sequencing Maja Kosecka-Strojek 1,* , Mariola Wolska 1, Dorota Zabicka˙ 2 , Ewa Sadowy 3 and Jacek Mi˛edzobrodzki 1 1 Department of Microbiology, Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University, 30-387 Krakow, Poland; [email protected] (M.W.); [email protected] (J.M.) 2 Department of Molecular Microbiology, National Medicines Institute, 00-725 Warsaw, Poland; [email protected] 3 Department of Epidemiology and Clinical Microbiology, National Medicines Institute, 00-725 Warsaw, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-12-664-6365 Received: 13 October 2020; Accepted: 9 November 2020; Published: 11 November 2020 Abstract: Streptococci and enterococci are significant opportunistic pathogens in epidemiology and infectious medicine. High genetic and taxonomic similarities and several reclassifications within genera are the most challenging in species identification. The aim of this study was to identify Streptococcus and Enterococcus species using genetic and phenotypic methods and to determine the most discriminatory identification method. Thirty strains recovered from clinical samples representing 15 streptococcal species, five enterococcal species, and four nonstreptococcal species were subjected to bacterial identification by the Vitek® 2 system and Sanger-based sequencing methods targeting the 16S rRNA, sodA, tuf, rpoB, and recA genes. Phenotypic methods allowed the identification of 10 streptococcal strains, five enterococcal strains, and four nonstreptococcal strains (Leuconostoc, Granulicatella, and Globicatella genera). The combination of sequencing methods allowed the identification of 21 streptococcal strains, five enterococcal strains, and four nonstreptococcal strains. -

The Ecology, Epidemiology and Virulence of Enterococcus
Microbiology (2009), 155, 1749–1757 DOI 10.1099/mic.0.026385-0 Review The ecology, epidemiology and virulence of Enterococcus Katie Fisher and Carol Phillips Correspondence University of Northampton, School of Health, Park Campus, Boughton Green Road, Northampton Katie Fisher NN2 7AL, UK [email protected] Enterococci are Gram-positive, catalase-negative, non-spore-forming, facultative anaerobic bacteria, which usually inhabit the alimentary tract of humans in addition to being isolated from environmental and animal sources. They are able to survive a range of stresses and hostile environments, including those of extreme temperature (5–65 6C), pH (4.5”10.0) and high NaCl concentration, enabling them to colonize a wide range of niches. Virulence factors of enterococci include the extracellular protein Esp and aggregation substances (Agg), both of which aid in colonization of the host. The nosocomial pathogenicity of enterococci has emerged in recent years, as well as increasing resistance to glycopeptide antibiotics. Understanding the ecology, epidemiology and virulence of Enterococcus speciesisimportant for limiting urinary tract infections, hepatobiliary sepsis, endocarditis, surgical wound infection, bacteraemia and neonatal sepsis, and also stemming the further development of antibiotic resistance. Introduction Taxonomy For many years Enterococcus species were believed to be The genus Enterococcus consists of Gram-positive, catalase- harmless to humans and considered unimportant med- negative, non-spore-forming, facultative anaerobic bacteria ically. Because they produce bacteriocins, Enterococcus that can occur both as single cocci and in chains. species have been used widely over the last decade in the Enterococci belong to a group of organisms known as food industry as probiotics or as starter cultures (Foulquie lactic acid bacteria (LAB) that produce bacteriocins Moreno et al., 2006). -
Comparative Genomics of Enterococcus Spp. Isolated from Bovine Feces Alicia G
Beukers et al. BMC Microbiology (2017) 17:52 DOI 10.1186/s12866-017-0962-1 RESEARCHARTICLE Open Access Comparative genomics of Enterococcus spp. isolated from bovine feces Alicia G. Beukers1,2†, Rahat Zaheer2†, Noriko Goji3, Kingsley K. Amoako3, Alexandre V. Chaves1, Michael P. Ward1 and Tim A. McAllister2* Abstract Background: Enterococcus is ubiquitous in nature and is a commensal of both the bovine and human gastrointestinal (GI) tract. It is also associated with clinical infections in humans. Subtherapeutic administration of antibiotics to cattle selects for antibiotic resistant enterococci in the bovine GI tract. Antibiotic resistance genes (ARGs) may be present in enterococci following antibiotic use in cattle. If located on mobile genetic elements (MGEs) their dissemination between Enterococcus species and to pathogenic bacteria may be promoted, reducing the efficacy of antibiotics. Results: We present a comparative genomic analysis of twenty-one Enterococcus spp. isolated from bovine feces including Enterococcus hirae (n =10),Enterococcus faecium (n =3),Enterococcus villorum (n =2),Enterococcus casseliflavus (n =2),Enterococcus faecalis (n =1),Enterococcus durans (n =1),Enterococcus gallinarum (n =1)and Enterococcus thailandicus (n = 1). The analysis revealed E. faecium and E. faecalis from bovine feces share features with human clinical isolates, including virulence factors. The Tn917 transposon conferring macrolide-lincosamide- streptogramin B resistance was identified in both E. faecium and E. hirae, suggesting dissemination of ARGs on MGEsmayoccurinthebovineGItract.AnE. faecium isolate was also identified with two integrative conjugative elements (ICEs) belonging to the Tn916 family of ICE, Tn916 and Tn5801, both conferring tetracycline resistance. Conclusions: This study confirms the presence of enterococci in the bovine GI tract possessing ARGs on MGEs, but the predominant species in cattle, E. -

Beneficial Effect of Bacteriocin-Producing Strain Enterococcus Durans ED 26E/7 in Model Experiment Using Broiler Rabbits
Original Paper Czech J. Anim. Sci., 62, 2017 (4): 168–177 doi: 10.17221/21/2016-CJAS Beneficial Effect of Bacteriocin-producing Strain Enterococcus durans ED 26E/7 in Model Experiment Using Broiler Rabbits Andrea Lauková1*, Monika Pogány Simonová1, Ľubica Chrastinová2, Anna Kandričáková1, Jana Ščerbová1, Iveta Plachá1, Klaudia Čobanová1, Zuzana Formelová2, Ľubomír Ondruška2, Gabriela Štrkolcová3, Viola Strompfová1 1Institute of Animal Physiology, Slovak Academy of Sciences, Košice, Slovak Republic 2National Agricultural and Food Centre, Lužianky, Slovak Republic 3University of Veterinary Medicine and Pharmacy, Košice, Slovak Republic *Correspoding author: [email protected] ABSTRACT Lauková A., Pogány Simonová M., Chrastinová Ľ., Kandričáková A., Ščerbová J., Plachá I., Čobanová K., Formelová Z., Ondruška Ľ., Štrkolcová G., Strompfová V. (2017): Beneficial effect of bacteriocin-producing strain Enterococcus durans ED 26E/7 in model experiment using broiler rabbits. Czech J. Anim. Sci., 62, 168–177. From the aspect of probiotic properties and bacteriocins, Enterococcus faecium belongs to the most frequently studied species among enterococci. This study deals with testing the strain of the species Enterococcus durans ED 26E/7 in broiler rabbits. The strain ED 26E/7 isolated from ewes lump cheese produces an antimicrobial sub- stance durancin. Forty-eight post-weaned rabbits (aged 5 weeks) of both sexes were divided into experimental group (EG) and control group (CG) per 24 animals each, and kept in standard cages, two animals per cage. EG group rabbits were additionally administered the ED 26E/7 strain (500 µl/animal/day) into water for 21 days. CG group rabbits were fed a commercial feed. The experiment lasted 42 days. Faeces and blood samples were taken on days 0–1 (experiment onset), 21 (after a 3-week application), and 42 (3 weeks after ED 26E/7 strain cessation). -

Cycle 36 Organism 1
P.O. Box 131375, Bryanston, 2074 Ground Floor, Block 5 Bryanston Gate, 170 Curzon Road Bryanston, Johannesburg, South Africa 804 Flatrock, Buiten Street, Cape Town, 8001 www.thistle.co.za Tel: +27 (011) 463 3260 Fax: +27 (011) 463 3036 Fax to Email: + 27 (0) 86-557-2232 e-mail : [email protected] Please read this section first The HPCSA and the Med Tech Society have confirmed that this clinical case study, plus your routine review of your EQA reports from Thistle QA, should be documented as a “Journal Club” activity. This means that you must record those attending for CEU purposes. Thistle will not issue a certificate to cover these activities, nor send out “correct” answers to the CEU questions at the end of this case study. The Thistle QA CEU No is: MT-2014/004. Each attendee should claim THREE CEU points for completing this Quality Control Journal Club exercise, and retain a copy of the relevant Thistle QA Participation Certificate as proof of registration on a Thistle QA EQA. MICROBIOLOGY LEGEND CYCLE 36 ORGANISM 1 Enterococcus casseliflavus Enterococcus is a genus of lactic acid bacteria of the phylum Firmicutes. Enterococci are Gram-positive cocci that often occur in pairs (diplococci) or short chains, and are difficult to distinguish from streptococci on physical characteristics alone. Two species are common commensal organisms in the intestines of humans: E. faecalis (90-95%) and E. faecium (5-10%) but are also important pathogens responsible for serious infections. Rare clusters of infections occur with other species, including E. casseliflavus, E. gallinarum, and E. -

Resistance Mechanisms and Inflammatory Bowel Disease 10.1515/Med-2020-0032 Present Multi-Resistant E
Open Med. 2020; 15: 211-224 Research Article Michaela Růžičková, Monika Vítězová, Ivan Kushkevych* The characterization of Enterococcus genus: resistance mechanisms and inflammatory bowel disease https://doi.org/ 10.1515/med-2020-0032 present multi-resistant E. faecium belongs to a different received September 18, 2019; accepted February 5, 2020 taxon than the original strains isolated from animals. This separation must have happened around 75 years ago and Abstract: The constantly growing bacterial resistance is being connected to antibiotic usage in clinical practice. against antibiotics is recently causing serious problems This clade can be distinguished by its increased number in the field of human and veterinary medicine as well as of mobile genetic elements, metabolic alternations and in agriculture. The mechanisms of resistance formation hypermutability [1]. All of these attributes led to the devel- and its preventions are not well explored in most bacterial opment of a flexible genome, which is now able to easily genera. The aim of this review is to analyse recent litera- adapt to the changes of the surroundings [2,3]. ture data on the principles of antibiotic resistance forma- It is also necessary to mention some basic informa- tion in bacteria of the Enterococcus genus. Furthermore, tion about morphological diversity, physiological and bio- the habitat of the Enterococcus genus, its pathogenicity chemical characteristics and taxonomy in order to under- and pathogenicity factors, its epidemiology, genetic and stand the resistance mechanisms completely. Biochemical molecular aspects of antibiotic resistance, and the rela- features must especially be mentioned since they play a tionship between these bacteria and bowel diseases are huge role in the high increase of resistance in these bac- discussed. -

Urinary Tract Infection Caused by Enterococcus Spp: Risk Factors and Mortality
Urinary Tract Infection Caused By Enterococcus Spp: Risk Factors And Mortality. An Observational Study. Elisa Alvarez-Artero CAUPA Amaia Campo Nuñez CAUPA Inmaculada Garcia Garcia CAUSA Moises Garcia Bravo CAUPA Olia Cores CAUSA Inmaculada Galindo Perez CAUPA Jose Pendones CAUSA Amparo Lopez Bernus CAUSA Moncef Belhassen-Garcia ( [email protected] ) Hospital Universitario de Salamanca https://orcid.org/0000-0002-2230-1256 Javier Pardo Lleidas Marques de Valdecilla Research article Keywords: Urinary tract infection, Enterococcus spp., Mortality, Bacteremia, Immunosuppression Posted Date: June 10th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-32100/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/18 Version of Record: A version of this preprint was published at Revista Clínica Española (English Edition) on May 1st, 2021. See the published version at https://doi.org/10.1016/j.rceng.2020.09.004. Page 2/18 Abstract Background Urinary tract infections (UTIs) are frequently caused by Enterococcus spp. We aim to dene the risk factors involved in UTIs caused by Enterococcus. Determine the overall mortality and predictive risk factors. Methods A retrospective in-patients study was conducted with bacteriemic UTIs caused by Enterococcus spp. We compared bacteriemic UTIs caused by Enterococcus spp. vs. a random sample of 100 in-patients with bacteriemic UTIs caused by others enterobacteria. Results We found 106 in-patients with UTIs caused by Enterococcus spp., 51 of whom had concomitant positive blood cultures. Distribution by species was: 83% E. faecalis and 17% E. faecium, with a Charlson comorbidity index of 5.9 ± 2.9. -

FOOD and HEALTH Isolation and Identification of Staphylococcus
FOOD and HEALTH E-ISSN 2602-2834 Food Health 6(3), 141-159 (2020) • https://doi.org/10.3153/FH20016 Research Article Isolation and identification of Staphylococcus aureus obtained from cheese samples Elif Bozcal Cite this article as: Bozcal, E. (2020). Isolation and identification of Staphylococus aureus obtained from cheese samples. Food and Health, 6(3), 151-159. https://doi.org/10.3153/FH20016 Istanbul University, Faculty of Science, ABSTRACT Department of Biology, Basic and Industrial Microbiology Section, 34134, Milk and dairy products including cheese are one of the most significant food commodities in Istanbul, Turkey terms of the food industry. However, a contaminated food product could conduce a variety of food borne bacterial infections. Although Staphylococcus aureus is known as normal flora members of ORCID IDs of the authors: the humans, it`s often isolated from the community and hospital-acquired infections. Therefore, E.B. 0000-0003-2836-778X investigation of Staphylococcus aureus from cheese samples was aimed in this study. A total of nineteen (n=19) white cheese was collected from various outdoor markets in Istanbul. All cheese samples were evaluated quantitatively. Phenotypic identification tests including Gram staining, oxidase, catalase, mannitol, and DNase were performed. The presumptive Staphylococcus aureus colonies (n=47) were analyzed by the 16S rRNA PCR and sequencing. And the sequences were Submitted: 13.11.2019 deposited into the National Center for Biotechnology Information. According to the nucleotide Revision requested: 09.02.2020 BLAST analysis, a total of 47 Staphylococaceae and Enterococcaceae members including Staph- Last revision received: 28.02.2020 ylococcus aureus (n=3), Staphylococcus carnosus (n=1), Macrococcus caseolyticus (n=1), Enter- ococcus faecalis (n=25), Enterococcus faecium (n=12), Enterococcus durans (n=4), and Entero- Accepted: 29.02.2020 coccus gallinarum (n=1) were identified. -
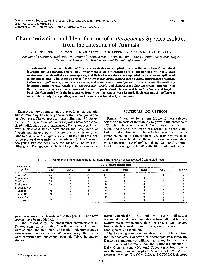
Characterization and Identification of Enterococcus Species Isolated from the Intestines of Animals L
INTERNATIONALJOURNAL OF SYSTEMATICBACTERIOLOGY, July 1987, p. 257-259 Vol. 37, No. 3 0020-7713/87/030257-03$02.00/0 Copyright 0 1987, International Union of Microbiological Societies Characterization and Identification of Enterococcus Species Isolated from the Intestines of Animals L. A. DEVRIESE,'* A. VAN DE KERCKHOVE,l R. KILPPER-BALZ,* AND K. H. SCHLEIFER2 Faculty of Veterinary Medicine, University of Ghent, B-9000 Ghent, Belgium,' and Lehrstuhl fiir Mikrobiologie, Technische Universitat, Munich, Federal Republic of Germany2 Tests useful for the identification of Enterococcus strains were applied to a collection of isolates from animal intestines and to reference strains, all of which were capable of growth on 40% bile and in 6.5% NaCl. Most strains could be identified as known species, and their characteristics corresponded, with a few exceptions of minor importance, with those described for Enterococcus hirae, Enterococcus durans, Enterococcus mundtii, Enterococcus gallinarum, Enterococcus avium, and Enterococcus casseliflavus. However, some diagnostically important carbohydrate reactions of Enterococcus faecalis and Enterococcus faecium strains differed from those given in the species descriptions and in recent reports. Production of acid from D-raffinose and D-xylose by E.faecium varied with the host species from which the strains were isolated. E. durans and E. gallinarum were isolated only from poultry, whereas E. avium was found only in mammals. Enterococci are an important group of intestinal bacteria MATERIALS AND METHODS whose taxonomy has undergone important changes in the last few years. The enterococci (sensu Sherman [9]), Strep- Strains. A total of 264 strains (Table 1) were selected tococcus bovis, and Streptococcus equinus have been clas- solely on the basis of their ability to grow in the presence of sified traditionally as serological group D streptococci. -

Investigation Into the Influence of a Bacteriocin-Producing Enterococcus Strain on the Intestinal Microflora
Investigation into the influence of a bacteriocin-producing Enterococcus strain on the intestinal microflora Zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften an der Fakultät für Chemie und Biowissenschaften der Universität Karlsruhe genehmigte DISSERTATION von Agus Wijaya aus Palembang, Indonesien Referent: Prof. Dr. W.H. Holzapfel Co-Referent: Prof. Dr. W. Zumft Tag der mündlichen Prüfung: 02., 3., und 5. 12. 2003 ACKNOWLEDGMENT I would like to express my respect and appreciation to Prof. W.H. Holzapfel, Director of Institute of Hygiene and Toxicology in the Federal Research Centre for Nutrition (BFE), who has given me a recommendation needed in applying for DAAD (German Academic Exchange Service) scholarship. This recommendation has proven to be decisive in joint selection. I am very thankful for the chance he gave me to work in his excellent institute and for always being attentive when I needed his advice. Special thanks to Prof. Dr. W. Zumft for supporting my admission as Ph.D. student at the Fakultät für Chemie und Biowissenschaften, University of Karlsruhe and for his continued interest. My great gratitude comes to Dr. C.M.A.P. Franz, Head of the Molecular Microbiology Laboratory in BFE, for his dedicatory supervision and uncountable guidance throughout of my doctoral research. It would have been very difficult without his supervision and endless, valuable suggestions. I will always be grateful. I express my thank to Dr. Christian Neudecker who has given me valuable advice and guidance in preparing and implementing the animal experiment. My next round of thanks goes Mrs. Ingrid Specht and Ms. Anja Waldheim who has given me valuable technical assistance.