Guide to the Sterling Drug, Inc. Records
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identifying the Defendant in Products Liability Litigation: Alternate Theories for Compensating Injured Plaintiffs
International Journal of Business and Social Science Vol. 9 • No. 8 • August 2018 doi:10.30845/ijbss.v9n8p2 Identifying the Defendant in Products Liability Litigation: Alternate Theories for Compensating Injured Plaintiffs Richard J. Hunter, Jr. Professor of Legal Studies Seton Hall University United States John H. Shannon Professor of Legal Studies Seton Hall University United States Henry J. Amoroso Associate Professor of Legal Studies Seton Hall University United States Abstract A spirited public policy debate has raged in the United States for more than four decades revolving around the responsibility of drug manufacturers for their products that cause injury to unsuspecting plaintiffs. An important part of the debate centers around a drug—DES—that was marketed as a benefit to women in preventing miscarriages. Some interesting or "alternate" theories for assessing liability have received attention in the generic area of products liability. This article will discuss three of the most prominent and still controversial theories—alternative liability, enterprise liability, and market share liability. In addition, the article will include several case studies exemplifying the legal principles discussed — some coming to very difficult conclusions — regarding the efficacy and application of these theories to particular facts developed during litigation. Keywords: Alternative Liability; Enterprise Liability; Market Share Liability 1. Introduction DES (Diethylstilbestrol, a synthetic form of the female hormone estrogen) litigation in the United States has been both extensive and controversial. Forty years ago, at the height of the DES crisis, in a seminal law review article in the Fordham Law Review, Sheiner (1978) estimated that the number of women who took DES during pregnancy ranged from 1 1/2 million to 3 million. -

ANNNNNNNNNNNNNNNNNNNN 100A 006 Left Eye Input Right Eye Input
US 20190175049A1 ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2019 /0175049 A1 Welling ( 43 ) Pub . Date : Jun . 13 , 2019 ( 54 ) TECHNIQUES FOR ANALYZING (52 ) U . S . CI. NON -VERBAL MARKERS OF CONDITIONS CPC . .. A61B 5 /04842 (2013 . 01 ) ; A61B 5 / 7289 USING ELECTROPHYSIOLOGICAL DATA (2013 . 01) ; A61B 5 /0478 ( 2013 .01 ) ; A61B 5 /7225 ( 2013. 01 ) ; G06N 20 / 10 (2019 .01 ) (71 ) Applicant: Massachusetts Institute of Technology , Cambridge , MA (US ) ( 57 ) ABSTRACT (72 ) Inventor : Caroline Welling, Hanover, NH (US ) Embodiments related to analyzing brain activity of a subject to identify signs associated with binocular rivalry . Sensed ( 21 ) Appl. No. : 16 / 206, 639 electrical activity of a subject' s brain is received over a time period while the subject is exposed to a visual stimulus. The ( 22 ) Filed : Nov. 30 , 2018 sensed electrical activity comprises a first frequency band Related U . S . Application Data associated with a first frequency of a first image presented to the subject ' s left eye , a second frequency band associated (60 ) Provisional application No .62 / 593 , 535, filed on Dec . with a second frequency of a second image presented to the 1 , 2017 subject ' s right eye . A set of events in the time period is determined based on the frequency bands, wherein an event Publication Classification is associated with a change from a previous perceptual event (51 ) Int. Ci. to a new perceptual event. A metric for the subject is A61B 5 /0484 ( 2006 .01 ) determined based on the set of events . The metric is ana A61B 5 /00 ( 2006 .01 ) lyzed to determine whether the subject exhibits signs asso GO6N 20 / 10 (2006 .01 ) ciated with a condition that is associated with binocular A61B 5 /0478 ( 2006 .01 ) rivalry . -

Sulphonmethane, Sulphonal, Diethylsulpho
is a combination of amylene hydrate and chloral hydrate, superior to the corresponding compounds of other elements. while chloralose, a combination of chloral hydrate and glucose, Experience has shown that, in the main, these claims were un- partakes of the action of morphin and is rather expensive. founded, though many, even now, claim that strontium bro- Chloretone, a more recent product, is not entirely devoid of mid disturbs the stomach less than the corresponding sodium danger and is not always so certain in its action as chloral or potassium salt. Another claim that is frequently made by hydrate, while butyl chloral hydrate, or crotón chloral hydrate, manufacturers of nostrums, like "Peacock's Bromides," is that is one of the older compounds that has been found wanting and they use "chemically pure" salts. Exactly what is meant by is now little used. Of the official compounds of this group we this claim is difficult to say, but the Pharmacopeia gives us a have: number of readily applied tests by which the salts themselves Chloralamid and Paraldehyd. may be tested. The manufacturers of nostrums, on the other Chlobalformamidum.—TJ. S.—Chloralformamid. Chlorala- hand, not infrequently add the very substances that are consid- mid. This has practically the same action as therapeutic ered contaminations. doses of chloral hydrate, the latter being formed in the body by {To be continued.) decomposition of chloralformamid. Average dose: 1 gm. (15 grains). Paraldehtdum.—TJ. S.—Paraldehyd is slower in its action transparent liquid, slower in its action than chloral hydrate, but also safer. It has the disadvantage of a persistently dis- A NEW NEEDLE HOLDER. -

V. : Civil Action No. DKC 14-3607
Case 8:14-cv-03607-DKC Document 47 Filed 07/29/15 Page 1 of 73 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MARYLAND : MALLINCKRODT INC. : v. : Civil Action No. DKC 14-3607 : UNITED STATES FOOD AND DRUG ADMINISTRATION, et al. : MEMORANDUM OPINION Plaintiff Mallinckrodt Inc. (“Mallinckrodt”), a pharmaceutical manufacturer, brings this action seeking judicial review of actions taken by the United States Food and Drug Administration (“FDA”), regarding its methylphenidate hydrochloride extended-release tablets, a generic version of the brand-name drug Concerta. Presently pending and ready for review in this action are several motions: (1) a motion to dismiss filed by Defendants FDA and United States of America (“Defendants”) (ECF No. 30); (2) a motion to compel production of the administrative record filed by Mallinckrodt (ECF No. 31); (3) a motion for summary judgment filed by Mallinckrodt (ECF No. 34); and (2) two motions to seal filed by Mallinckrodt (ECF Nos. 4 and 20). The relevant issues have been briefed, and the court now rules, no hearing being deemed necessary. Local Rule 105.6. For the following reasons, Defendants’ motion to dismiss will be granted in part, and summary judgment will be granted in part Case 8:14-cv-03607-DKC Document 47 Filed 07/29/15 Page 2 of 73 against Plaintiff. Mallinckrodt’s motion to compel production of the administrative record will be denied as moot, and Mallinckrodt’s motions to seal will be denied. I. Background A. Statutory and Regulatory Background The FDA regulates the approval, manufacture, sale, and labeling of prescription drugs. -

Determination of Barbiturates and 11-Nor-9-Carboxy-Δ9-THC in Urine
Determination of Barbiturates and 11-Nor-9-carboxy- 9-THC in Urine using Automated Disposable Pipette Extraction (DPX) and LC/MS/MS Fred D. Foster, Oscar G. Cabrices, John R. Stuff, Edward A. Pfannkoch GERSTEL, Inc., 701 Digital Dr. Suite J, Linthicum, MD 21090, USA William E. Brewer Department of Chemistry and Biochemistry, University of South Carolina, 631 Sumter St. Columbia, SC 29208, USA KEYWORDS DPX, LC/MS/MS, Sample Preparation, High Throughput Lab Automation ABSTRACT This work demonstrates the use of disposable pipette extraction (DPX) as a fast and automated sample preparation technique for the determination of barbiturates and 11-nor-9- carboxy- 9-THC (COOH-THC) in urine. Using a GERSTEL MultiPurpose Sampler (MPS) with DPX option coupled to an Agilent 6460 LC-MS/MS instrument, 8 barbiturates and COOH-THC were extracted and their concentrations determined. The resulting average cycle time of 7 min/ sample, including just-in-time sample preparation, enabled high throughput screening. Validation results show that the automated DPX-LC/ AppNote 1/2013 MS/MS screening method provides adequate sensitivity for all analytes and corresponding internal standards that were monitored. Lower limits of quantitation (LLOQ) were found to be 100 ng/mL for the barbiturates and 10 ng/mL for COOH-THC and % CVs were below 10 % in most cases. INTRODUCTION The continuously growing quantity of pain management sample extract for injection [1-2]. The extraction of the drugs used has increased the demand from toxicology Barbiturates and COOH-THC is based on the DPX-RP- laboratories for more reliable solutions to monitor S extraction method described in an earlier Application compliance in connection with substance abuse and/or Note detailing monitoring of 49 Pain Management diversion. -
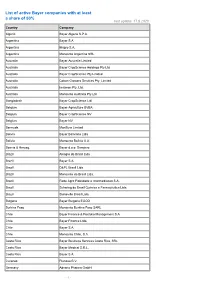
List of Active Bayer Companies with at Least a Share of 50% Last Update: 17.8.2020 Country Company
List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Algeria Bayer Algerie S.P.A. Argentina Bayer S.A. Argentina Biagro S.A. Argentina Monsanto Argentina SRL Australia Bayer Australia Limited Australia Bayer CropScience Holdings Pty Ltd Australia Bayer CropScience Pty Limited Australia Cotton Growers Services Pty. Limited Australia Imaxeon Pty. Ltd. Australia Monsanto Australia Pty Ltd Bangladesh Bayer CropScience Ltd. Belgium Bayer Agriculture BVBA Belgium Bayer CropScience NV Belgium Bayer NV Bermuda MonSure Limited Bolivia Bayer Boliviana Ltda Bolivia Monsanto Bolivia S.A. Bosnia & Herzeg. Bayer d.o.o. Sarajevo Brazil Alkagro do Brasil Ltda Brazil Bayer S.A. Brazil D&PL Brasil Ltda Brazil Monsanto do Brasil Ltda. Brazil Rede Agro Fidelidade e Intermediacao S.A. Brazil Schering do Brasil Química e Farmacêutica Ltda. Brazil Stoneville Brasil Ltda. Bulgaria Bayer Bulgaria EOOD Burkina Faso Monsanto Burkina Faso SARL Chile Bayer Finance & Portfolio Management S.A. Chile Bayer Finance Ltda. Chile Bayer S.A. Chile Monsanto Chile, S.A. Costa Rica Bayer Business Services Costa Rica, SRL Costa Rica Bayer Medical S.R.L. Costa Rica Bayer S.A. Curacao Pianosa B.V. Germany Adverio Pharma GmbH - 1 - List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Germany AgrEvo Verwaltungsgesellschaft mbH Germany Alcafleu Management GmbH & Co. KG Germany BGI Deutschland GmbH Germany Bayer 04 Immobilien GmbH Germany Bayer 04 Leverkusen Fußball GmbH Germany Bayer 04 Leverkusen -

Steven Sanders, Et Al. V. Bayer (AG) Aktiengesellschaft, Et Al. 03-CV-1546-Second Consolidated Amended Complaint
Q,^.IGiNAL &&,iN 2i 4- L UNITED STATES DISTRICT COURT SOUTHERN DISTRICT OF NEW YORK -_DOF x Consolidated Civil Action IN RE BAYER AG SECURITIES No. 03 CV 1546 (WHP) LITIGATION Class Action x 71 :n = SECOND CONSOLIDATED AMENDED COMPLAINT Jury Trial Demand MILBERG WEISS BERSHAD & SCHULMAN LLP Melvyn I. Weiss (MW-1392) Michael C . Spencer (MS-8874) Lee A. Weiss (LW-1130) Jennifer Sclar (JS-7313) One Pennsylvania Plaza New York, NY 10119 (212) 594-5300 Attorneys for Lead Plaintiff January 14, 2005 i • ' Table of Contents Page SUMMARY OF CLAIMS ...............................................................................................................2 JURISDICTION AND VENUE ...................................................................................................... 8 A. General Jurisdiction and Venue ............................................................................... 8 B. Subject Matter Jurisdiction Over Claims of Foreign Purchasers on Foreign Exchanges ................................................................................................................ 8 PARTIES .......................................................................................................................................12 RELEVANT EVENTS ..................................................................................................................14 A. Background of the Statin Market ...........................................................................14 B. FDA Approval and Introduction of Baycol; Early Safety Warnings -

Intravenous Anesthetics a Drug That Induces Reversible Anesthesia the State of Loss of Sensations, Or Awareness
DR.MED. ABDELKARIM ALOWEIDI AL-ABBADI ASSOCIATE PROF. FACULTY OF MEDICINE THE UNIVERSITY OF JORDAN Goals of General Anesthesia Hypnosis (unconsciousness) Amnesia Analgesia Immobility/decreased muscle tone (relaxation of skeletal muscle) Reduction of certain autonomic reflexes (gag reflex, tachycardia, vasoconstriction) Intravenous Anesthetics A drug that induces reversible anesthesia The state of loss of sensations, or awareness. Either stimulates an inhibitory neuron, or inhibits an excitatory neuron. Organs are divided according to blood perfusion: High- perfusion organs (vessel-rich); brain takes up disproportionately large amount of drug compared to less perfused areas (muscles, fat, and vessel-poor groups). After IV injection, vessel-rich group takes most of the available drug Drugs bound to plasma proteins are unavailable for uptake by an organ After highly perfused organs are saturated during initial distribution, the greater mass of the less perfused organs continue to take up drug from the bloodstream. As plasma concentration falls, some drug leaves the highly perfused organs to maintain equilibrium. This redistribution from the vessel-rich group is responsible for termination of effect of many anesthetic drugs. Compartment Model Inhibitory channels : GABA-A channels ( the main inhibitory receptor). Glycine channels. Excitatory channels : Neuronal nicotic. NMDA. Rapid onset (mainly unionized at physiological pH) High lipid solubility Rapid recovery, no accumulation during prolonged infusion Analgesic -

Brief of Consumers Union of United States, Inc., As Amicus Curiae in Support of Petitioners, Riegel & Riegel V
Georgetown University Law Center Scholarship @ GEORGETOWN LAW 2007 Brief of Consumers Union of United States, Inc., as Amicus Curiae in Support of Petitioners, Riegel & Riegel v. Medtronic, Inc., No. 06-179 (U.S. Aug. 27, 2007) Lisa Heinzerling Georgetown University Law Center Docket No. 06-179 This paper can be downloaded free of charge from: http://scholarship.law.georgetown.edu/scb/40 This open-access article is brought to you by the Georgetown Law Library. Posted with permission of the author. Follow this and additional works at: http://scholarship.law.georgetown.edu/scb Part of the Consumer Protection Law Commons, and the Torts Commons No. 06-179 ~ ~!~! GLE~a~K IN THE £ upreme ¢£ourt of nite tate CHARLES R. RIEGEL AND DONNA S. RIEGEL, Petitioners V. MEDTRONIC, INC., Respondent ON WRIT OF CERTIORARI TO THE UNITED STATES COURT OF APPEALS FOR THE SECOND CIRCUIT BRIEF OF CONSUMERS UNION OF UNITED STATES, INC., AS AMICUS CURIAE IN SUPPORT OF PETITIONERS LISA HEINZERLING Counsel of Record 600 New Jersey Ave., N.W. Washington, D.C. 20001 (202) 662-9115 MARK SAVAGE Consumers Union of United States, Inc. 1535 Mission Street San Francisco, CA 94103-2512 Counsel for Amicus Consumers Union of United States, Inc. WILSON-EPES PRINTINGCO., INC. - (202) 789-0096 - WASHINGTON, D. C. 20002 QUESTION PRESENTED Whether the express preemption provision of the Medical Device Amendments to the Food, Drug, and Cosmetic Act, 21 U.S.C. § 360k(a), preempts state-law claims seeking damages for injuries caused by medical devices that received premarket approval from the Food and Drug Administration. -

International Classification of Diseases
INTERNATIONAL CLASSIFICATION OF DISEASES MANUAL OF THE INTERNATIONAL STATISTICAL CLASSIFICATION OF DISEASES, INJURIES, AND CAUSES OF DEATH Based on the Recommendations of the Eighth Revision Conference, 1965, and Adopted by the Nineteenth World Health Assembly Volume 2 ALPHABETICAL INDEX WORLD HEALTH ORGANIZATION GENEVA 1969 Volume 1 Introduction List of Three-digit Categories Tabular List of Inclusions and Four-digit Sub- categories Medical Certification and Rules for Classification Special Lists for Tabulation Definitions and Recommendations Regulations Volume 2 Alphabetical Index PRINTED IN ENGLAND CONTENTS Introduction Page General arrangement of the Index ....................................... VIII Main sections ............................................................... VIII Structure ..................................................................... IX Code numbzrs .............................................................. x Primary and secondary conditions. ................................... x Multiple diagnoses. ........................................................ XI Spelling....................................................................... XI Order of listing ............................................................. Conventions used in the Index ........................................... XII Parentheses. ................................................................. XII Cross-referexes ........................................................... XI1 Abbreviation NEC. ...................................................... -

Notes to the Consolidated Financial Statements of the Bayer Group
Five-Year Summary Bayer Annual Report 2018 Five-Year Summary 2014 2015 2016 2017 2018 Bayer Group (€ million) Sales 41,339 46,085 34,943 35,015 39,586 EBITDA1 8,315 9,573 8,801 8,563 10,266 1 EBITDA before special items 8,685 10,256 9,318 9,288 9,547 1 EBITDA margin before special items (in %) 21.0 22.3 26.7 26.5 24.1 EBIT1 5,395 6,241 5,738 5,903 3,914 1 EBIT before special items 5,833 7,060 6,826 7,130 6,480 Income before income taxes 4,414 5,236 4,773 4,577 2,318 Net income (from continuing and discontinued operations) 3,426 4,110 4,531 7,336 1,695 1 Earnings per share (from continuing and discontinued operations) (€) 4.14 4.97 5.44 8.29 1.80 1 Core earnings per share (from continuing operations) (€) 5.89 6.82 6.67 6.64 5.94 Net cash provided by operating activities (from continuing and discontinued operations) 5,810 6,890 9,089 8,134 7,917 Net financial debt 19,612 17,449 11,778 3,595 35,679 2 Capital expenditures (as per segment table) 2,484 2,554 2,627 2,418 2,564 Bayer AG Total dividend payment 1,861 2,067 2,233 2,402 2,611 Dividend per share (€) 2.25 2.50 2.70 2.80 2.80 Innovation Research and development expenses (€ million) 3,537 4,274 4,405 4,504 5,246 Ratio of R&D expenses to sales – Pharmaceuticals (%) 15.6 16.0 16.7 16.2 15.5 Ratio of R&D expenses to sales – Crop Science (%) 10.3 10.7 11.7 11.7 13.0 Employees in research and development3 13,900 14,753 14,213 14,041 17,275 Employees 3 Number of employees (Dec. -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine